Stromal protein βig-h3 reprogrammes tumour microenvironment in pancreatic cancer
- PMID: 30415234
- PMCID: PMC6580775
- DOI: 10.1136/gutjnl-2018-317570
Stromal protein βig-h3 reprogrammes tumour microenvironment in pancreatic cancer
Abstract
Objective: Pancreatic cancer is associated with an abundant stromal reaction leading to immune escape and tumour growth. This massive stroma drives the immune escape in the tumour. We aimed to study the impact of βig-h3 stromal protein in the modulation of the antitumoural immune response in pancreatic cancer.
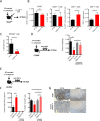
Design: We performed studies with p48-Cre;KrasG12D, pdx1-Cre;KrasG12D;Ink4a/Arffl/fl, pdx1-Cre;KrasG12D; p53R172H mice and tumour tissues from patients with pancreatic ductal adenocarcinoma (PDA). Some transgenic mice were given injections of anti-βig-h3, anti-CD8, anti-PD1 depleting antibodies. Tumour growth as well as modifications in the activation of local immune cells were analysed by flow cytometry, immunohistochemistry and immunofluorescence. Tissue stiffness was measured by atomic force microscopy.
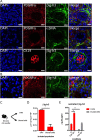
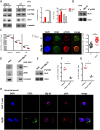
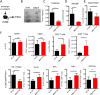
Results: We identified βig-h3 stromal-derived protein as a key actor of the immune paracrine interaction mechanism that drives pancreatic cancer. We found that βig-h3 is highly produced by cancer-associated fibroblasts in the stroma of human and mouse. This protein acts directly on tumour-specific CD8+ T cells and F4/80 macrophages. Depleting βig-h3 in vivo reduced tumour growth by enhancing the number of activated CD8+ T cell within the tumour and subsequent apoptotic tumour cells. Furthermore, we found that targeting βig-h3 in established lesions released the tissue tension and functionally reprogrammed F4/80 macrophages in the tumour microenvironment.
Conclusions: Our data indicate that targeting stromal extracellular matrix protein βig-h3 improves the antitumoural response and consequently reduces tumour weight. Our findings present βig-h3 as a novel immunological target in pancreatic cancer.
Keywords: immune response; pancreatic cancer; t lymphocytes.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


Comment in
-
Immunosuppressive βig-h3 links tumour stroma and dysfunctional T cells in pancreatic cancer.Gut. 2019 Apr;68(4):581. doi: 10.1136/gutjnl-2018-317735. Epub 2018 Dec 7. Gut. 2019. PMID: 30530506 No abstract available.
-
A deeper understanding of the tumor microenvironment in pancreatic cancer: the key to developing effective immunotherapies.Ann Transl Med. 2019 Apr;7(8):162. doi: 10.21037/atm.2019.03.14. Ann Transl Med. 2019. PMID: 31168443 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
