Long-Term Efficacy and Safety of Hizentra® in Patients with Primary Immunodeficiency in Japan, Europe, and the United States: a Review of 7 Phase 3 Trials
- PMID: 30415311
- PMCID: PMC6292970
- DOI: 10.1007/s10875-018-0560-5
Long-Term Efficacy and Safety of Hizentra® in Patients with Primary Immunodeficiency in Japan, Europe, and the United States: a Review of 7 Phase 3 Trials
Abstract
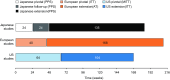
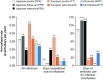
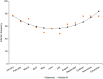
Many patients with primary immunodeficiency (PID) require immunoglobulin G (IgG) replacement therapy, delivered as intravenous IgG (IVIG) or subcutaneous IgG (SCIG). We aim to identify trends in efficacy and safety that would not be evident in individual studies of small patient numbers. Seven open-label, Phase 3, prospective, multicenter studies of the efficacy and safety of Hizentra® (a SCIG), conducted in Japan, Europe, and the US were summarized. Overall, 125 unique patients received 15,013 weekly infusions during a total observation period of 250.9 patient-years. Mean weekly doses of Hizentra® were 83.22-221.3 mg/kg body weight; infusion rates per patient (total body rate) were 25.2-49.3 mL/h across studies. The rates of infections and serious bacterial infections were 3.10 and 0.03 events per patient/year, respectively. Annualized rates of days hospitalized due to infection, out of work/school, and prophylactic antibiotic use were 0.95, 5.14, and 36.78 per patient, respectively. For the equivalent monthly dose, weekly Hizentra® SCIG administration resulted in expectedly-increased serum IgG trough levels in patients switching from IVIG, and maintained levels in patients switching from previous SCIG. Adverse events (AEs) totaled 5039 (events/infusion 0.094-0.773), almost all of which were mild/moderate. Three thousand one hundred ninety-seven were considered treatment-related, the most common of which were injection site reactions (2919 events; 0.001-0.592 AEs per infusion). Systemic AEs were very uncommon. The results from these seven studies indicate that Hizentra® therapy was both efficacious and well tolerated during long-term treatment. This is particularly important in patients with PID, who may require lifelong IgG replacement therapy.
Keywords: IVIG; Immunoglobulin G replacement therapy; SCIG; primary antibody deficiencies; primary immunodeficiencies.
Conflict of interest statement
Conflict of Interest
Stephen Jolles has participated in advisory boards, trials, projects, and has been a speaker with Baxalta, CSL Behring, Shire, Thermofisher, Swedish Orphan Biovitrum, Biotest, Binding Site, Grifols, BPL, Octapharma, LFB, GSK, Weatherden, Zarodex, Sanofi, and UCB Pharma. Mikhail Rojavin, John-Philip Lawo, and Michael A. Tortorici are employees of CSL Behring. Robert Nelson Jr. reported no conflicts of interest. Richard L. Wasserman has been Consultant and Investigator for BPL, Korean Green Cross, Prometic, Therapure, and Shire, and has been a speaker for Shire. Michael Borte has no potential conflicts of interest. Kohsuke Imai has been a Consultant for CSL Behring, a Speaker for CSL Behring, Novartis, The Japan Blood Products Organization. Hirokazu Kanegane has received research grant support from CSL Behring and the Japanese Blood Products Organization, and has been a Consultant for CSL Behring.
Figures

Similar articles
-
Treatment Satisfaction with Subcutaneous Immunoglobulin Replacement Therapy in Patients with Primary Immunodeficiency: a Pooled Analysis of Six Hizentra® Studies.J Clin Immunol. 2018 Nov;38(8):886-897. doi: 10.1007/s10875-018-0562-3. Epub 2018 Nov 21. J Clin Immunol. 2018. PMID: 30465179 Free PMC article.
-
Efficacy and safety of a new 20% immunoglobulin preparation for subcutaneous administration, IgPro20, in patients with primary immunodeficiency.J Clin Immunol. 2010 Sep;30(5):734-45. doi: 10.1007/s10875-010-9423-4. Epub 2010 May 8. J Clin Immunol. 2010. PMID: 20454851 Free PMC article. Clinical Trial.
-
Efficacy and safety of IgPro20, a subcutaneous immunoglobulin, in Japanese patients with primary immunodeficiency diseases.J Clin Immunol. 2014 Feb;34(2):204-11. doi: 10.1007/s10875-013-9985-z. Epub 2014 Feb 7. J Clin Immunol. 2014. PMID: 24504846 Free PMC article. Clinical Trial.
-
Subcutaneous immunoglobulin replacement therapy with Hizentra, the first 20% SCIG preparation: a practical approach.Adv Ther. 2011 Jul;28(7):521-33. doi: 10.1007/s12325-011-0036-y. Epub 2011 Jun 14. Adv Ther. 2011. PMID: 21681653 Review.
-
Home therapy with subcutaneous immunoglobulins for patients with primary immunodeficiency diseases.Transfus Apher Sci. 2012 Jun;46(3):315-21. doi: 10.1016/j.transci.2012.03.022. Epub 2012 Apr 12. Transfus Apher Sci. 2012. PMID: 22503304 Review.
Cited by
-
Pediatric subset of primary immunodeficiency patients treated with SCIG: post hoc analysis of SHIFT and IBIS pooled data.Allergy Asthma Clin Immunol. 2020 Sep 9;16:80. doi: 10.1186/s13223-020-00478-2. eCollection 2020. Allergy Asthma Clin Immunol. 2020. PMID: 32944034 Free PMC article.
-
Monoclonal antibody and protein therapeutic formulations for subcutaneous delivery: high-concentration, low-volume vs. low-concentration, high-volume.MAbs. 2023 Jan-Dec;15(1):2285277. doi: 10.1080/19420862.2023.2285277. Epub 2023 Nov 27. MAbs. 2023. PMID: 38013454 Free PMC article. Review.
-
Long-term efficacy, safety, and tolerability of a subcutaneous immunoglobulin 16.5% (cutaquig®) in the treatment of patients with primary immunodeficiencies.Clin Exp Immunol. 2022 Dec 15;210(2):91-103. doi: 10.1093/cei/uxac092. Clin Exp Immunol. 2022. PMID: 36208448 Free PMC article.
-
Reducing Delays in Diagnosing Primary Immunodeficiency Through the Development and Implementation of a Clinical Decision Support Tool: Protocol for a Quality Improvement Project.JMIR Res Protoc. 2022 Jan 4;11(1):e32635. doi: 10.2196/32635. JMIR Res Protoc. 2022. PMID: 34587114 Free PMC article.
-
Healthcare resource utilization and costs in immunodeficient patients receiving subcutaneous Ig: Real-world evidence from France.PLoS One. 2025 Jan 24;20(1):e0313694. doi: 10.1371/journal.pone.0313694. eCollection 2025. PLoS One. 2025. PMID: 39854356 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

