Low-Dose Radiation Conditioning Enables CAR T Cells to Mitigate Antigen Escape
- PMID: 30415658
- PMCID: PMC6225039
- DOI: 10.1016/j.ymthe.2018.09.008
Low-Dose Radiation Conditioning Enables CAR T Cells to Mitigate Antigen Escape
Abstract
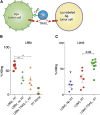
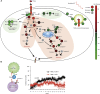
CD19 chimeric antigen receptors (CARs) have demonstrated great efficacy against a range of B cell malignancies. However, antigen escape and, more generally, heterogeneous antigen expression pose a challenge to applying CAR therapy to a wide range of cancers. We find that low-dose radiation sensitizes tumor cells to immune rejection by locally activated CAR T cells. In a model of pancreatic adenocarcinoma heterogeneously expressing sialyl Lewis-A (sLeA), we show that not only sLeA+ but also sLeA- tumor cells exposed to low-dose radiation become susceptible to CAR therapy, reducing antigen-negative tumor relapse. RNA sequencing analysis of low-dose radiation-exposed tumors reveals the transcriptional signature of cells highly sensitive to TRAIL-mediated death. We find that sLeA-targeted CAR T cells produce TRAIL upon engaging sLeA+ tumor cells, and eliminate sLeA- tumor cells previously exposed to systemic or local low-dose radiation in a TRAIL-dependent manner. These findings enhance the prospects for successfully applying CAR therapy to heterogeneous solid tumors. Local radiation is integral to many tumors' standard of care and can be easily implemented as a CAR conditioning regimen.
Keywords: CAR T cell; antigen escape; pancreatic cancer; radiation; sialyl Lewis-A.
Copyright © 2018 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Brentjens R.J., Latouche J.B., Santos E., Marti F., Gong M.C., Lyddane C., King P.D., Larson S., Weiss M., Rivière I., Sadelain M. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat. Med. 2003;9:279–286. - PubMed
-
- Wilkie S., van Schalkwyk M.C., Hobbs S., Davies D.M., van der Stegen S.J., Pereira A.C., Burbridge S.E., Box C., Eccles S.A., Maher J. Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. J. Clin. Immunol. 2012;32:1059–1070. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

