Granulocyte macrophage-colony stimulating factor: A key modulator of renal mononuclear phagocyte plasticity
- PMID: 30415915
- PMCID: PMC6401212
- DOI: 10.1016/j.imbio.2018.10.007
Granulocyte macrophage-colony stimulating factor: A key modulator of renal mononuclear phagocyte plasticity
Abstract
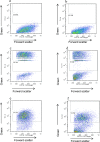
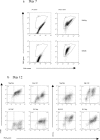
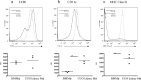
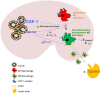
Macrophage-colony stimulating factor (M-CSF) and granulocyte macrophage-colony stimulating factor (GM-CSF) play key roles in the differentiation of macrophages and dendritic cells (DCs). We examined the effect of treatment with M-CSF-containing macrophage medium or GM-CSF-containing DC medium upon the phenotype of murine bone marrow-derived macrophages and DCs. Culture of macrophages for 5 days in DC medium reduced F4/80 expression and increased CD11c expression with cells effectively stimulating T cell proliferation in a mixed lymphocyte reaction. DC medium treatment of macrophages significantly reduced phagocytosis of both apoptotic cells and latex beads and strongly induced the expression of the chemokine receptor CCR7 known to be involved in DC trafficking to lymph nodes. Lysates of obstructed murine kidneys expressed both M-CSF and GM-CSF though M-CSF expression was dominant (M-CSF:GM-CSF ratio ∼30:1). However, combination treatment with both M-CSF and GM-CSF (ratio 30:1) indicated that small amounts of GM-CSF skewed macrophages towards a DC-like phenotype. To determine whether macrophage phenotype might be modulated in vivo we tracked CD45.1+ bone marrow-derived macrophages intravenously administered to CD45.2+ mice with unilateral ureteric obstruction. Flow cytometry of enzyme dissociated kidneys harvested 3 days later indicated CD11c and MHC Class II upregulation by adoptively transferred CD45.1+ cells with CD45.1+ cells evident in draining renal lymph nodes. Our data suggests that GM-CSF modulates mononuclear phagocyte plasticity, which likely promotes resolution of injury and healing in the injured kidney.
Keywords: DC; GM-CSF; Inflammation; M-CSF; Macrophage; Mononuclear phagocyte.
Copyright © 2018 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures






References
-
- Anders H.J., Ryu M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 2011;80:915. - PubMed
-
- Brocheriou I., Maouche S., Durand H., Braunersreuther V., Le Naour G., Gratchev A., Koskas F., Mach F., Kzhyshkowska J., Ninio E. Antagonistic regulation of macrophage phenotype by M-CSF and GM-CSF: implication in atherosclerosis. Atherosclerosis. 2011;214:316. - PubMed
-
- Cao Q., Wang Y., Wang X.M., Lu J., Lee V.W., Ye Q., Nguyen H., Zheng G., Zhao Y., Alexander S.I., Harris D.C. Renal F4/80+ CD11c+ mononuclear phagocytes display phenotypic and functional characteristics of macrophages in health and in adriamycin nephropathy. J. Am. Soc. Nephrol. 2015;26:349. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

