Inhibition of Estrogen Signaling Reduces the Incidence of BRCA1-associated Mammary Tumor Formation
- PMID: 30416390
- PMCID: PMC6216038
- DOI: 10.7150/ijbs.28142
Inhibition of Estrogen Signaling Reduces the Incidence of BRCA1-associated Mammary Tumor Formation
Abstract
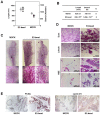
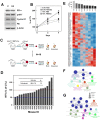
BRCA1-deficient breast cancer is a very well-known hereditary cancer. However, except for resection of normal mammary glands and ovaries, there is no acceptable measure for proactively preventing tumor development. Importantly, inherited BRCA1 mutations are closely associated with tumors in hormone-responsive tissues. Here, we examined the effects of estrogen on the accumulation of genetic instabilities upon loss of BRCA1, and assessed the contribution of estrogen signaling to the incidence and progression of Brca1-mutated mammary tumors. Our in vitro studies showed that treatment of BRCA1-depleted breast cancer cells with estrogen induced proliferation. Additionally, estrogen reduced the ability of these BRCA1-knockdown cells to sense radiation-induced DNA damage and also facilitated G1/S progression. Moreover, long-term treatment of Brca1-mutant (Brca1co/coMMTV-Cre) mice with the selective estrogen receptor (ER)-α degrader, fulvestrant, decreased the tumor formation rate from 64% to 36%, and also significantly reduced mammary gland density in non-tumor-bearing mice. However, in vivo experiments showed that fulvestrant treatment did not alter the progression of ER-positive Brca1-mutant tumors, which were frequently identified in the aged population and showed less aggressive tendencies. These findings enhance our understanding of how ER-α signaling contributes to BRCA1-deficient mammary tumors and provide evidence suggesting that targeted inhibition of ER-α signaling may be useful for the prevention of BRCA1-mutated breast cancer.
Keywords: BRCA1; cancer prevention; estrogen; fulvestrant.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S. et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. - PubMed
-
- Venkitaraman R. Triple-negative/basal-like breast cancer: clinical, pathologic and molecular features. Expert Rev Anticancer Ther. 2010;10:199–207. - PubMed
-
- Valentin MD, da Silva SD, Privat M, Alaoui-Jamali M, Bignon YJ. Molecular insights on basal-like breast cancer. Breast Cancer Res Treat. 2012;134:21–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

