Modulation of Antibody Responses to the V1V2 and V3 Regions of HIV-1 Envelope by Immune Complex Vaccines
- PMID: 30416503
- PMCID: PMC6212562
- DOI: 10.3389/fimmu.2018.02441
Modulation of Antibody Responses to the V1V2 and V3 Regions of HIV-1 Envelope by Immune Complex Vaccines
Abstract
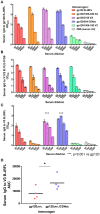
Prophylactic HIV vaccines must elicit antibodies (Abs) against the virus envelope glycoproteins (Env) to effectively prevent HIV infection. We investigated a vaccine platform that utilizes immune complexes made of Env proteins gp120 and monoclonal Abs (mAbs) against different gp120 epitopes. We previously observed alterations in V3 antigenicity upon formation of certain gp120/mAb complexes and demonstrated the ability of these complexes to modulate the elicitation of V3 Ab responses. However, the effects on the V1V2 domain, an important target for Abs that correlate with vaccine-induced protection against HIV, have not been studied, nor have immune complex vaccines made with non-B subtype Env. This study compared subtypes B (JRFL) and CRF_01.AE (A244) Env gp120 proteins in complex with selected gp120-specific mAbs. Allosteric and antigenic changes were detected on these immune complexes, indicating that gp120/mAb interaction induces alterations on the Env surface that may modify the Env immunogenic properties. To evaluate this idea, mice were immunized with gp120/mAb complexes or their uncomplexed gp120 counterparts. The overall serum IgG titers elicited against gp120 were comparable, but a marked skewing toward V1V2 or V3 was evident and dependent on the gp120 strain and the specificity of the mAb used to form the complexes. Compared with uncomplexed gp120JRFL, gp120JRFL complexed with CD4bs or V1V2 mAbs, but not with C2 or V3 mAbs, elicited V3 Abs of greater titers and breadth, and Abs more capable of neutralizing tier 1 virus. Epitope mapping revealed a shift to a more conserved site in the V3 crown. However, the complexes did not enhance V1V2 Ab response, and the elicited V1V2 Abs were not cross-reactive. This profile contrasts with Ab responses to gp120A244/mAb complexes. Notably, gp120A244/mAb complexes induced higher levels of V1V2 Abs with some cross-reactivity, while also stimulating weak or strain-specific V3 Abs. Sera from gp120A244/mAb complex-immunized animals displayed no measurable virus neutralization but did mediate Ab-dependent cellular phagocytosis, albeit at levels similar to that induced by gp120A244 alone. These data indicate the potential utility of immune complexes as vaccines to shape Ab responses toward or away from Env sites of interest.
Keywords: HIV; antibody; envelope; immune complex; vaccine.
Figures




Similar articles
-
Elicitation of broadly reactive antibodies against glycan-modulated neutralizing V3 epitopes of HIV-1 by immune complex vaccines.Vaccine. 2013 Nov 4;31(46):5413-21. doi: 10.1016/j.vaccine.2013.09.010. Epub 2013 Sep 16. Vaccine. 2013. PMID: 24051158 Free PMC article.
-
The use of immune complex vaccines to enhance antibody responses against neutralizing epitopes on HIV-1 envelope gp120.Vaccine. 2009 Dec 11;28(2):352-60. doi: 10.1016/j.vaccine.2009.10.040. Epub 2009 Oct 29. Vaccine. 2009. PMID: 19879224 Free PMC article.
-
Improving immunogenicity of HIV-1 envelope gp120 by glycan removal and immune complex formation.Vaccine. 2011 Nov 8;29(48):9064-74. doi: 10.1016/j.vaccine.2011.09.057. Epub 2011 Sep 23. Vaccine. 2011. PMID: 21945958 Free PMC article.
-
Vaccine-induced V1V2-specific antibodies control and or protect against infection with HIV, SIV and SHIV.Curr Opin HIV AIDS. 2019 Jul;14(4):309-317. doi: 10.1097/COH.0000000000000551. Curr Opin HIV AIDS. 2019. PMID: 30994501 Free PMC article. Review.
-
Improving on nature: focusing the immune response on the V3 loop.Hum Antibodies. 2005;14(3-4):69-72. Hum Antibodies. 2005. PMID: 16720976 Review.
Cited by
-
P2X1 Selective Antagonists Block HIV-1 Infection through Inhibition of Envelope Conformation-Dependent Fusion.J Virol. 2020 Feb 28;94(6):e01622-19. doi: 10.1128/JVI.01622-19. Print 2020 Feb 28. J Virol. 2020. PMID: 31852781 Free PMC article.
-
Signal peptide exchange alters HIV-1 envelope antigenicity and immunogenicity.Front Immunol. 2024 Sep 24;15:1476924. doi: 10.3389/fimmu.2024.1476924. eCollection 2024. Front Immunol. 2024. PMID: 39380992 Free PMC article.
-
Vaccination with immune complexes modulates the elicitation of functional antibodies against HIV-1.Front Immunol. 2023 Oct 3;14:1271686. doi: 10.3389/fimmu.2023.1271686. eCollection 2023. Front Immunol. 2023. PMID: 37854587 Free PMC article.
-
Targeting HIV Env immunogens to B cell follicles in nonhuman primates through immune complex or protein nanoparticle formulations.NPJ Vaccines. 2020 Aug 5;5(1):72. doi: 10.1038/s41541-020-00223-1. eCollection 2020. NPJ Vaccines. 2020. PMID: 32802411 Free PMC article.
-
Antibody Responses Elicited by Immunization with BG505 Trimer Immune Complexes.J Virol. 2019 Sep 30;93(20):e01188-19. doi: 10.1128/JVI.01188-19. Print 2019 Oct 15. J Virol. 2019. PMID: 31375582 Free PMC article.
References
-
- Robb ML, Rerks-Ngarm S, Nitayaphan S, Pitisuttithum P, Kaewkungwal J, Kunasol P, et al. . Risk behaviour and time as covariates for efficacy of the HIV vaccine regimen ALVAC-HIV (vCP1521) and AIDSVAX B/E: a post-hoc analysis of the Thai phase 3 efficacy trial RV 144. Lancet Infect Dis. (2012) 12:531–7. 10.1016/S1473-3099(12)70088-9 - DOI - PMC - PubMed
-
- Gottardo R, Bailer RT, Korber BT, Gnanakaran S, Phillips J, Shen X, et al. . Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial. PLoS ONE (2013) 8:e75665. 10.1371/journal.pone.0075665 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

