The Role of Glucocorticoid Receptors in Podocytes and Nephrotic Syndrome
- PMID: 30417008
- PMCID: PMC6224173
- DOI: 10.11131/2018/101323
The Role of Glucocorticoid Receptors in Podocytes and Nephrotic Syndrome
Abstract
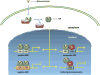
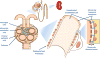
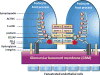
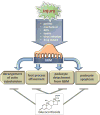
Glucocorticoid receptor (GC), a founding member of the nuclear hormone receptor superfamily, is a glucocorticoid-activated transcription factor that regulates gene expression and controls the development and homeostasis of human podocytes. Synthetic glucocorticoids are the standard treatment regimens for proteinuria (protein in the urine) and nephrotic syndrome (NS) caused by kidney diseases. These include minimal change disease (MCD), focal segmental glomerulosclerosis (FSGS), membranous nephropathy (MN) and immunoglobulin A nephropathy (IgAN) or subsequent complications due to diabetes mellitus or HIV infection. However, unwanted side effects and steroid-resistance remain major issues for their long-term use. Furthermore, the mechanism by which glucocorticoids elicit their renoprotective activity in podocyte and glomeruli is poorly understood. Podocytes are highly differentiated epithelial cells that contribute to the integrity of kidney glomerular filtration barrier. Injury or loss of podocytes leads to proteinuria and nephrotic syndrome. Recent studies in multiple experimental models have begun to explore the mechanism of GC action in podocytes. This review will discuss progress in our understanding of the role of glucocorticoid receptor and glucocorticoids in podocyte physiology and their renoprotective activity in nephrotic syndrome.
Keywords: Glucocorticoid receptor; Nephrotic syndrome; Podocyte; focal segmental glomerulosclerosis.
Conflict of interest statement
Competing Interests The authors declare no competing interests.
Figures

Similar articles
-
Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome.Nat Med. 2011 Jan;17(1):117-22. doi: 10.1038/nm.2261. Epub 2010 Dec 12. Nat Med. 2011. PMID: 21151138 Free PMC article.
-
Immunoexpression of podocyte-associated proteins in acquired human glomerulopathies with nephrotic syndrome.Pol J Pathol. 2006;57(1):17-21. Pol J Pathol. 2006. PMID: 16739878
-
Krüppel-Like Factor 15 Mediates Glucocorticoid-Induced Restoration of Podocyte Differentiation Markers.J Am Soc Nephrol. 2017 Jan;28(1):166-184. doi: 10.1681/ASN.2015060672. Epub 2016 Jun 10. J Am Soc Nephrol. 2017. PMID: 27288011 Free PMC article.
-
Genetic basis of adult-onset nephrotic syndrome and focal segmental glomerulosclerosis.Front Med. 2017 Sep;11(3):333-339. doi: 10.1007/s11684-017-0564-1. Epub 2017 Aug 3. Front Med. 2017. PMID: 28776307 Review.
-
Regulation of TRPC6 ion channels in podocytes - Implications for focal segmental glomerulosclerosis and acquired forms of proteinuric diseases.Acta Physiol Hung. 2015 Sep;102(3):241-51. doi: 10.1556/036.102.2015.3.2. Acta Physiol Hung. 2015. PMID: 26551740 Review.
Cited by
-
Role of p90RSK in Kidney and Other Diseases.Int J Mol Sci. 2019 Feb 23;20(4):972. doi: 10.3390/ijms20040972. Int J Mol Sci. 2019. PMID: 30813401 Free PMC article. Review.
-
CXCL16/ERK1/2 pathway regulates human podocytes growth, migration, apoptosis and epithelial mesenchymal transition.Mol Med Rep. 2022 Jun;25(6):212. doi: 10.3892/mmr.2022.12728. Epub 2022 May 6. Mol Med Rep. 2022. PMID: 35514316 Free PMC article.
-
How immunosuppressive drugs may directly target podocytes in glomerular diseases.Pediatr Nephrol. 2022 Jul;37(7):1431-1441. doi: 10.1007/s00467-021-05196-4. Epub 2021 Jul 9. Pediatr Nephrol. 2022. PMID: 34244853 Review.
-
Inhibition of the glucocorticoid receptor attenuates proteinuric kidney diseases in multiple species.Nephrol Dial Transplant. 2024 Jun 28;39(7):1181-1193. doi: 10.1093/ndt/gfad254. Nephrol Dial Transplant. 2024. PMID: 38037533 Free PMC article.
-
p90RSK modulates inter-and intracellular signaling in kidney diseases.Front Cell Dev Biol. 2025 Jun 5;13:1593914. doi: 10.3389/fcell.2025.1593914. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40538979 Free PMC article. Review.
References
-
- Bledsoe RK, Montana VG, Stanley TB, et al. Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition. Cell. 2002;110(1):93–105. - PubMed
-
- Bledsoe RK, Stewart EL, Pearce KH. Structure and Function of the Glucocorticoid Receptor Ligand Binding Domain. Vitamins & Hormones. 2004;68:49–91. - PubMed
-
- Vandevyver S, Dejager L, Libert C. On the Trail of the Glucocorticoid Receptor: Into the Nucleus and Back. Traffic. 2012;13(3):364–374. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
