Improvement in Patient-Reported Outcomes (Dermatology Life Quality Index and the Psoriasis Symptoms and Signs Diary) with Guselkumab in Moderate-to-Severe Plaque Psoriasis: Results from the Phase III VOYAGE 1 and VOYAGE 2 Studies
- PMID: 30417277
- PMCID: PMC6513809
- DOI: 10.1007/s40257-018-0396-z
Improvement in Patient-Reported Outcomes (Dermatology Life Quality Index and the Psoriasis Symptoms and Signs Diary) with Guselkumab in Moderate-to-Severe Plaque Psoriasis: Results from the Phase III VOYAGE 1 and VOYAGE 2 Studies
Abstract
Background: Health-related quality of life (HRQoL) may be markedly impaired in patients with moderate-to-severe psoriasis.
Objectives: Our objectives were to compare improvements in Dermatology Life Quality Index (DLQI) and Psoriasis Symptoms and Signs Diary (PSSD) scores between patients receiving guselkumab compared with placebo or adalimumab and to correlate these improvements with skin clearance.
Methods: Pooled phase III VOYAGE 1 and VOYAGE 2 data were evaluated through week 24. At baseline, patients were randomized to guselkumab 100 mg, placebo, or adalimumab 40 mg. At week 16, patients receiving placebo switched to guselkumab. Assessment measures included DLQI percent change from baseline, DLQI 0/1, DLQI minimal clinically important difference (MCID), individual domain scores, PSSD symptoms and signs score = 0, DLQI association with PSSD, Investigator's Global Assessment (IGA), and Psoriasis Area and Severity Index (PASI).
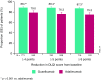
Results: Significantly greater improvements from baseline DLQI were observed with guselkumab versus placebo (weeks 8 and 16) and versus adalimumab (week 24; p < 0.001). The proportion of patients achieving DLQI 0/1 ("no impact") at week 24 was higher with guselkumab than with adalimumab (58.9 vs. 40.2%; p < 0.001), and more patients attained a ≥ 4-point reduction in DLQI (MCID) at this timepoint (p < 0.001). Changes in individual DLQI domains were significantly greater for patients receiving guselkumab than for those receiving adalimumab, and among patients with individual baseline domain scores = 3 or 6 (severest impact), more guselkumab recipients than those receiving adalimumab achieved a score = 0 across all domains at week 24. DLQI 0/1 scores were associated with a PSSD symptom or sign score = 0 (no impact) and greater improvement of PASI and IGA (week 24).
Conclusions: Pooled VOYAGE 1/VOYAGE 2 data demonstrated that guselkumab was superior to adalimumab in improving HRQoL, which was associated with greater skin clearance.
Clinical trial registration: NCT02207231 and NCT02207244.
Conflict of interest statement
Conflict of interest
A Armstrong has received grant funding from AbbVie, Eli Lilly, and Janssen, and consulting fees/honorarium from AbbVie, Janssen, Eli Lilly, Leo Pharma, Modernizing Medicine, Novartis, Ortho Dermatologics, Regeneron, Sanofi, and Science 37. K. Reich has served as an advisor and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Affibody, Almirall, Amgen, Biogen, Boenhringer Ingelheim, Celgene, Covagen, Eli Lilly, Forward Pharma, Frensenius Medical Care, GlaxoSmtihKline, Janssen, Janssen-Cilag, Kyowa Kirin, Leo Pharma, Medac, Merck Sharp & Dohme, Novartis, Miltenyi Biotec, Ocean Pharma, Pfizer, Regeneron, Samsung Bioepis, Sanofi, Takeda, UCB, Valeant, and Xenoport. P. Foley has received research grants from Abbott/AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Novartis, Shering-Plough/MSD, and Wyeth/Pfizer; served as an advisory board member for Abbott/AbbVie, Amgen, Biogen Idec, Celgene, Eli Lilly, Shering-Plough/MSD, Galderma, Janssen, Leo Pharma/Peplin, Novartis, Sanofi Genzyme, Sun Pharma, UCB, and Wyeth/Pfizer; received travel support for investigators’ meetings for clinical trials as the Principal Investigator; and received speaker bureau fees from 3 M/iNova/Valeant, Abbott/AbbVie, Biogen Idec, Celgene, Eli Lilly, Galderma, GSK/Stiefel, Janssen, Leo Pharma/Peplin, Novartis, Shering-Plough/MSD, and Wyeth/Pfizer. K. Papp has served as a consultant and/or scientific advisor and/or investigator and/or scientific officer and/or speaker for AbbVie, Akros, Allergan, Amgen, Anacor, Arcutis, Astrellas, AstraZeneca, Baxalta, Baxter, Boehringer Ingelheim, BMS, CanFite, Celgene, Coherus, Demira, Dow Pharma, Eli Lilly, Forward Pharam, Galderma, Genentech, GlaxoSmithKline, Janssen, Kyowa Hakko Kirin, Leo Pharma, Medimmune, Meiji Seiki Pharma, Merck Sharp & Dohme, Merck-Serono, Mitsubishi Pharma, Novartis, Pfizer, Regeneron, Roche, Sanofi-Aventis/Genzyme, Takeda, UCB, and Valeant. C. Han, M. Song, Y.-K. Shen, and Y. You are employees of Janssen Research & Development, LLC. and own stock in Johnson & Johnson, of which Janssen is a subsidiary.
Ethical approval/informed consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. All patients provided written informed consent prior to any study-related procedures.
Figures

References
-
- Finlay AY, Khan GK. Dermatology Life Quality Index. http://www.bad.org.uk. Accessed Apr 1992.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

