Preferences for in-person disclosure: Patients declining telephone disclosure characteristics and outcomes in the multicenter Communication Of GENetic Test Results by Telephone study
- PMID: 30417332
- PMCID: PMC6453119
- DOI: 10.1111/cge.13474
Preferences for in-person disclosure: Patients declining telephone disclosure characteristics and outcomes in the multicenter Communication Of GENetic Test Results by Telephone study
Abstract
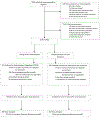
Telephone disclosure of cancer genetic test results is noninferior to in-person disclosure. However, how patients who prefer in-person communication of results differ from those who agree to telephone disclosure is unclear but important when considering delivery models for genetic medicine. Patients undergoing cancer genetic testing were recruited to a multicenter, randomized, noninferiority trial (NCT01736345) comparing telephone to in-person disclosure of genetic test results. We evaluated preferences for in-person disclosure, factors associated with this preference and outcomes compared to those who agreed to randomization. Among 1178 enrolled patients, 208 (18%) declined randomization, largely given a preference for in-person disclosure. These patients were more likely to be older (P = 0.007) and to have had multigene panel testing (P < 0.001). General anxiety (P = 0.007), state anxiety (P = 0.008), depression (P = 0.011), cancer-specific distress (P = 0.021) and uncertainty (P = 0.03) were higher after pretest counseling. After disclosure of results, they also had higher general anxiety (P = 0.003), depression (P = 0.002) and cancer-specific distress (P = 0.043). While telephone disclosure is a reasonable alternative to in-person disclosure in most patients, some patients have a strong preference for in-person communication. Patient age, distress and complexity of testing are important factors to consider and requests for in-person disclosure should be honored when possible.
Keywords: cancer genetic testing; genetic counseling; in-person disclosure preference; result disclosure; telephone disclosure.
© 2018 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
Figures
References
-
- Robson ME, Bradbury AR, Arun B, et al.: American Society of Clinical Oncology Policy Statement Update: Genetic and Genomic Testing for Cancer Susceptibility. J Clin Oncol 33:3660–7, 2015 - PubMed
-
- Gersh BJ, Maron BJ, Bonow RO, et al.: 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 124:2761–96, 2011 - PubMed
-
- Tchan M, Savige J, Patel C, et al.: KHA-CARI Autosomal Dominant Polycystic Kidney Disease Guideline: Genetic Testing for Diagnosis. Semin Nephrol 35:545–549 e2, 2015 - PubMed
-
- (ACMG) ACoMGaG: Policy Statement: Points to Consider in the Clinical Application of Genomic Sequencing, 2012 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

