Clinicopathologic features of anaplastic myxopapillary ependymomas
- PMID: 30417460
- PMCID: PMC7444646
- DOI: 10.1111/bpa.12673
Clinicopathologic features of anaplastic myxopapillary ependymomas
Abstract
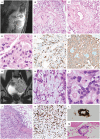
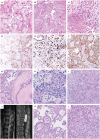
Myxopapillary ependymomas (MPE) are considered benign (World Health Organization (WHO) grade I) neoplasms with favorable prognosis. However, malignant behavior occurs in a small subset. To our knowledge, only five anaplastic MPEs have been reported without consensus on diagnostic criteria. We retrieved 14 anaplastic MPEs from the pathology archives of six institutions. Each tumor included at least two of the following features: ≥5 mitoses per 10 high power fields, Ki-67 labeling index (LI) ≥10%, microvascular proliferation (MVP) and spontaneous necrosis. These features were typically encountered in the foci of hypercellularity and reduced mucin. There were eight male and six female patients (age range 6-57 years, median = 16.5). Ten tumors displayed anaplasia at initial resection, and 4 were anaplastic at a second surgery for recurrence (ranging from 9 months to 14 years following initial resection). The Ki-67 LI ranged between 8% and 40% in the anaplastic foci and <3% in the foci of classic MPE. There was documented cerebrospinal fluid (CSF) dissemination in seven cases, recurrence following an anaplastic diagnosis in three cases and bone or soft tissue invasion in two cases. One patient suffered lung metastases. Two cases evaluated by targeted next-generation sequencing and one evaluated by fluorescence in situ hybridization (FISH) showed nonspecific chromosomal gains. We conclude that although rare, anaplastic MPE occurs in both pediatric and adult patients, similar to other ependymomas. At a minimum, closer follow-up is recommended, given the concern for aggressive biologic potential. Further study is needed to determine WHO grading criteria and genetic indicators of tumor progression.
Keywords: CSF dissemination; anaplastic transformation; malignant neoplasm; metastasis; microvascular proliferation; myxopapillary ependymoma; necrosis; recurrence.
© 2018 International Society of Neuropathology.
Figures
References
-
- Akpolat N, Bozlak N, Kazez A, Köseoğullari AA (2003) Sacrococcygeal extraspinal ependymoma: a case report. Turk J Pediatr 45:276–279. - PubMed
-
- Awaya H, Kaneko M, Amatya VJ, Takeshima Y, Oka S, Inai K (2003) Myxopapillary ependymoma with anaplastic features. Pathol Int 53:700–703. - PubMed
-
- Beschorner R, Wehrmann M, Ernemann U, Bonin M, Horber V, Oehl‐Jaschkowitz B et al (2007) Extradural ependymal tumor with myxopapillary and ependymoblastic differentiation in a case of Schinzel‐Giedion syndrome. Acta Neuropathol 113:339–346. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

