A Survey of Software Tool Utilization and Capabilities for Quantitative Systems Pharmacology: What We Have and What We Need
- PMID: 30417600
- PMCID: PMC6389347
- DOI: 10.1002/psp4.12373
A Survey of Software Tool Utilization and Capabilities for Quantitative Systems Pharmacology: What We Have and What We Need
Abstract
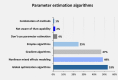
Quantitative systems pharmacology (QSP) is a rapidly emerging discipline with application across a spectrum of challenges facing the pharmaceutical industry, including mechanistically informed prioritization of target pathways and combinations in discovery, target population, and dose expansion decisions early in clinical development, and analyses for regulatory authorities late in clinical development. QSP's development has influences from physiologic modeling, systems biology, physiologically-based pharmacokinetic modeling, and pharmacometrics. Given a varied scientific heritage, a variety of tools to accomplish the demands of model development, application, and model-based analysis of available data have been developed. We report the outcome from a community survey and resulting analysis of how modelers view the impact and growth of QSP, how they utilize existing tools, and capabilities they need improved to further accelerate their impact on drug development. These results serve as a benchmark and roadmap for advancements to the QSP tool set.
© 2018 The Authors CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals, Inc. on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures

References
-
- Lalonde, R.L. et al Model‐based drug development. Clin. Pharmacol. Ther. 82, 21–32 (2007). - PubMed
-
- Bleicher, K.H. , Bohm, H.J. , Muller, K. & Alanine, A.I. Hit and lead generation: beyond high‐throughput screening. Nat. Rev. Drug Discov. 2, 369–378 (2003). - PubMed
-
- Huang, S.‐M. , Abernethy, D.R. , Wang, Y. , Zhao, P. & Zineh, I. The utility of modeling and simulation in drug development and regulatory review. J. Pharm. Sci. 102, 2912–2923 (2013). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

