Spinal plasticity with motor imagery practice
- PMID: 30417924
- PMCID: PMC6355716
- DOI: 10.1113/JP276694
Spinal plasticity with motor imagery practice
Abstract
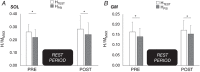
Key points: While a consensus has now been reached on the effect of motor imagery (MI) - the mental simulation of an action - on motor cortical areas, less is known about its impact on spinal structures. The current study, using H-reflex conditioning paradigms, examined the effect of a 20 min MI practice on several spinal mechanisms of the plantar flexor muscles. We observed modulations of spinal presynaptic circuitry while imagining, which was even more pronounced following an acute session of MI practice. We suggested that the small cortical output generated during MI may reach specific spinal circuits and that repeating MI may increase the sensitivity of the spinal cord to its effects. The short-term plasticity induced by MI practice may include spinal network modulation in addition to cortical reorganization.
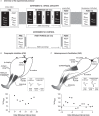
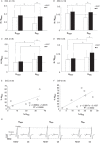
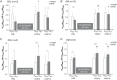
Abstract: Kinesthetic motor imagery (MI) is the mental simulation of a movement with its sensory consequences but without its concomitant execution. While the effect of MI practice on cortical areas is well known, its influence on spinal circuitry remains unclear. Here, we assessed plastic changes in spinal structures following an acute MI practice. Thirteen young healthy participants accomplished two experimental sessions: a 20 min MI training consisting of four blocks of 25 imagined maximal isometric plantar flexions, and a 20 min rest (control session). The level of spinal presynaptic inhibition was assessed by conditioning the triceps surae spinal H-reflex with two methods: (i) the stimulation of the common peroneal nerve that induced D1 presynaptic inhibition (HPSI response), and (ii) the stimulation of the femoral nerve that induced heteronymous Ia facilitation (HFAC response). We then compared the effects of MI on unconditioned (HTEST ) and conditioned (HPSI and HFAC ) responses before, immediately after and 10 min after the 20 min session. After resting for 20 min, no changes were observed on the recorded parameters. After MI practice, the amplitude of rest HTEST was unchanged, while HPSI and HFAC significantly increased, showing a reduction of presynaptic inhibition with no impact on the afferent-motoneuronal synapse. The current results revealed the acute effect of MI practice on baseline spinal presynaptic inhibition, increasing the sensitivity of the spinal circuitry to MI. These findings will help in understanding the mechanisms of neural plasticity following chronic practice.
Keywords: D1 presynaptic inhibition; H-reflex; heteronymous Ia facilitation; soleus; triceps surae.
© 2018 The Authors. The Journal of Physiology © 2018 The Physiological Society.
Figures
Similar articles
-
Vibration-induced depression in spinal loop excitability revisited.J Physiol. 2019 Nov;597(21):5179-5193. doi: 10.1113/JP278469. Epub 2019 Oct 3. J Physiol. 2019. PMID: 31429066
-
Immobilization induces changes in presynaptic control of group Ia afferents in healthy humans.J Physiol. 2008 Sep 1;586(17):4121-35. doi: 10.1113/jphysiol.2008.156547. Epub 2008 Jul 3. J Physiol. 2008. PMID: 18599534 Free PMC article.
-
Presynaptic control of group Ia afferents in relation to acquisition of a visuo-motor skill in healthy humans.J Physiol. 2005 Oct 1;568(Pt 1):343-54. doi: 10.1113/jphysiol.2005.089904. Epub 2005 Jul 28. J Physiol. 2005. PMID: 16051628 Free PMC article.
-
Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord.J Appl Physiol (1985). 2006 Dec;101(6):1776-82. doi: 10.1152/japplphysiol.00515.2006. Epub 2006 Sep 7. J Appl Physiol (1985). 2006. PMID: 16959909 Review.
-
Contributions to the understanding of gait control.Dan Med J. 2014 Apr;61(4):B4823. Dan Med J. 2014. PMID: 24814597 Review.
Cited by
-
Optimal stimulation parameters for spinal and corticospinal excitabilities during contraction, motor imagery and rest: A pilot study.PLoS One. 2020 Jun 22;15(6):e0235074. doi: 10.1371/journal.pone.0235074. eCollection 2020. PLoS One. 2020. PMID: 32569326 Free PMC article.
-
Stand Up to Excite the Spine: Neuromuscular, Autonomic, and Cardiometabolic Responses During Motor Imagery in Standing vs. Sitting Posture.Front Physiol. 2021 Nov 23;12:762452. doi: 10.3389/fphys.2021.762452. eCollection 2021. Front Physiol. 2021. PMID: 34887774 Free PMC article.
-
Motor Imagery Practice and Cognitive Processes.Front Psychol. 2020 Mar 3;11:394. doi: 10.3389/fpsyg.2020.00394. eCollection 2020. Front Psychol. 2020. PMID: 32194491 Free PMC article. No abstract available.
-
Inter-task transfer of force gains is facilitated by motor imagery.Front Neurosci. 2023 Aug 14;17:1228062. doi: 10.3389/fnins.2023.1228062. eCollection 2023. Front Neurosci. 2023. PMID: 37645373 Free PMC article.
-
Changes in corticospinal and spinal reflex excitability through functional electrical stimulation with and without observation and imagination of walking.Front Hum Neurosci. 2022 Sep 26;16:994138. doi: 10.3389/fnhum.2022.994138. eCollection 2022. Front Hum Neurosci. 2022. PMID: 36237950 Free PMC article.
References
-
- Achache V, Roche N, Lamy J‐C, Boakye M, Lackmy A, Gastal A, Quentin V & Katz R (2010). Transmission within several spinal pathways in adults with cerebral palsy. Brain 133, 1470–1483. - PubMed
-
- Aoyama T & Kaneko F (2011). The effect of motor imagery on gain modulation of the spinal reflex. Brain Res 1372, 41–48. - PubMed
-
- Baudry S & Enoka RM (2009). Influence of load type on presynaptic modulation of Ia afferent input onto two synergist muscles. Exp Brain Res 199, 83–88. - PubMed
-
- Bergmans J, Delwaide PJ & Gadea‐Ciria M (1978). Short‐latency effects of low‐threshold muscular afferent fibers on different motoneuronal pools of the lower limb in man. Exp Neurol 60, 380–385. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

