Integrated automated particle tracking microfluidic enables high-throughput cell deformability cytometry for red cell disorders
- PMID: 30417938
- PMCID: PMC7007699
- DOI: 10.1002/ajh.25345
Integrated automated particle tracking microfluidic enables high-throughput cell deformability cytometry for red cell disorders
Abstract
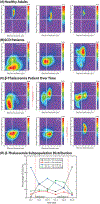
Investigating individual red blood cells (RBCs) is critical to understanding hematologic diseases, as pathology often originates at the single-cell level. Many RBC disorders manifest in altered biophysical properties, such as deformability of RBCs. Due to limitations in current biophysical assays, there exists a need for high-throughput analysis of RBC deformability with single-cell resolution. To that end, we present a method that pairs a simple in vitro artificial microvasculature network system with an innovative MATLAB-based automated particle tracking program, allowing for high-throughput, single-cell deformability index (sDI) measurements of entire RBC populations. We apply our technology to quantify the sDI of RBCs from healthy volunteers, Sickle cell disease (SCD) patients, a transfusion-dependent beta thalassemia major patient, and in stored packed RBCs (pRBCs) that undergo storage lesion over 4 weeks. Moreover, our system can also measure cell size for each RBC, thereby enabling 2D analysis of cell deformability vs cell size with single cell resolution akin to flow cytometry. Our results demonstrate the clear existence of distinct biophysical RBC subpopulations with high interpatient variability in SCD as indicated by large magnitude skewness and kurtosis values of distribution, the "shifting" of sDI vs RBC size curves over transfusion cycles in beta thalassemia, and the appearance of low sDI RBC subpopulations within 4 days of pRBC storage. Overall, our system offers an inexpensive, convenient, and high-throughput method to gauge single RBC deformability and size for any RBC population and has the potential to aid in disease monitoring and transfusion guidelines for various RBC disorders.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
CONFLICT OF INTEREST
Nothing to report.
Figures



References
-
- Yen RT, Fung YC. Effect of velocity distribution on red cell distribution in capillary blood vessels. Am J Physiol Heart Circ Physiol. 1978;235(2): H251–H257. - PubMed
-
- Stadler A, Linderkamp O. Flow behavior of neonatal and adult erythrocytes in narrow capillaries. Microvasc Res. 1989;37(3):267–279. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
