Changes in health-related quality of life with long-term eltrombopag treatment in adults with persistent/chronic immune thrombocytopenia: Findings from the EXTEND study
- PMID: 30417939
- PMCID: PMC6587804
- DOI: 10.1002/ajh.25348
Changes in health-related quality of life with long-term eltrombopag treatment in adults with persistent/chronic immune thrombocytopenia: Findings from the EXTEND study
Abstract
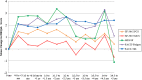
Patients with persistent/chronic immune thrombocytopenia (cITP) have low platelet counts, increased risk of bleeding and bruising, and often suffer from reduced health-related quality of life (HRQoL). cITP treatments may either improve HRQoL by increasing platelet counts or decrease it because of side effects. The open-label EXTEND study (June 2006 to July 2015) evaluated long-term safety, tolerability, and efficacy of eltrombopag (an oral thrombopoietin-receptor-agonist) in adults with cITP who completed a previous eltrombopag ITP trial. The final results of EXTEND were published and used to assess changes in patient-reported HRQoL over time and association between HRQoL and platelet response. Four validated HRQoL instruments were administered: SF-36v2 including physical component summary (PCS) and Mental Component Summary; Motivation and Energy Inventory Short Form (MEI-SF); Fatigue Subscale of FACIT (FACIT-Fatigue); and FACT-Thrombocytopenia Subscale Six-Item Extract (FACT-Th6). For the 302 patients enrolled, median duration of eltrombopag treatment was 2.37 years. All 4 HRQoL instruments demonstrated positive mean changes from baseline over time adjusted for patient baseline characteristics and rescue therapy use, and had positive association with platelet response (platelet count ≥30 × 109 /L; ≥50 × 109 /L; and ≥50 × 109 /L and >2 times baseline). Improvements from baseline started within 3 months and persisted through 5 years of treatment for FACIT-Fatigue and FACT-Th6 (P <.05 for nearly all time points); through 2.5 years for SF-36v2 PCS and less consistently for the MEI-SF. In conclusion, in addition to eltrombopag increasing platelet counts and reducing bleeding/bruising, it also alleviated fatigue, concerns about bleeding and bruising, and improved physical function in many patients, especially responders.
Keywords: bleeding; fatigue; platelets; thrombopoietin receptor agonist; vitality.
© 2018 The Authors. American Journal of Hematology published by Wiley Periodicals, Inc.
Conflict of interest statement
Abderrahim Khelif: Nothing to disclose.
Mansoor Saleh: Consultancy, research funding, and speakers bureau for GSK.
Abdulgabar Salama: Nothing to disclose.
Maria do Socorro O. Portella: Employed by Novartis.
Mei Sheng Duh: Research funding from Novartis, GSK, Bayer, Janssen, Eisai, Pfizer, Medtronic, Takeda, Novo Nordisk, Sanofi, Shire, Allergan, Taiho, CSL Behring.
Jasmina Ivanova: Research funding from Novartis, GSK, Teva, Lilly.
Kelly Grotzinger: Nothing to disclose.
Anuja Roy: Employed by Novartis.
James Bussel: Consultancy for Amgen, Novartis; research funding from Rigel Pharmaceuticals; membership on advisory committee for Momenta Pharmaceuticals, Novartis, Prophylix Pharma, Protalex, Rigel Pharmaceutical; patents and royalties for UpToDate; speakers bureau of Physicians Education Resource.
Figures
Similar articles
-
Validation of the FACIT-fatigue subscale, selected items from FACT-thrombocytopenia, and the SF-36v2 in patients with chronic immune thrombocytopenia.Qual Life Res. 2011 Dec;20(10):1737-44. doi: 10.1007/s11136-011-9912-9. Epub 2011 May 1. Qual Life Res. 2011. PMID: 21533818 Clinical Trial.
-
Eltrombopag for the treatment of children with persistent and chronic immune thrombocytopenia (PETIT): a randomised, multicentre, placebo-controlled study.Lancet Haematol. 2015 Aug;2(8):e315-25. doi: 10.1016/S2352-3026(15)00114-3. Epub 2015 Jul 28. Lancet Haematol. 2015. PMID: 26688484 Clinical Trial.
-
Eltrombopag for children with chronic immune thrombocytopenia (PETIT2): a randomised, multicentre, placebo-controlled trial.Lancet. 2015 Oct 24;386(10004):1649-58. doi: 10.1016/S0140-6736(15)61107-2. Epub 2015 Jul 28. Lancet. 2015. PMID: 26231455 Clinical Trial.
-
Eltrombopag: a review of its use in treatment-refractory chronic primary immune thrombocytopenia.Drugs. 2011 Jul 9;71(10):1333-53. doi: 10.2165/11207390-000000000-00000. Drugs. 2011. PMID: 21770480 Review.
-
Eltrombopag: A Review in Paediatric Chronic Immune Thrombocytopenia.Drugs. 2016 May;76(8):869-78. doi: 10.1007/s40265-016-0581-4. Drugs. 2016. PMID: 27151255 Review.
Cited by
-
Health-Related Quality of Life and Treatment of Older Adults with Acute Myeloid Leukemia: a Young International Society of Geriatric Oncology Review Paper.Curr Hematol Malig Rep. 2019 Dec;14(6):523-535. doi: 10.1007/s11899-019-00552-6. Curr Hematol Malig Rep. 2019. PMID: 31776773 Free PMC article. Review.
-
Assessing oral Health-Related quality of life in women undergoing chemotherapy for breast Cancer in Karachi Pakistan.Sci Rep. 2025 Mar 6;15(1):7846. doi: 10.1038/s41598-025-91533-8. Sci Rep. 2025. PMID: 40050631 Free PMC article.
-
Real-world impact of primary immune thrombocytopenia and treatment with thrombopoietin receptor agonists on quality of life based on patient-reported experience: Results from a questionnaire conducted in Switzerland, Austria, and Belgium.PLoS One. 2022 Apr 21;17(4):e0267342. doi: 10.1371/journal.pone.0267342. eCollection 2022. PLoS One. 2022. PMID: 35446925 Free PMC article.
-
Eltrombopag treatment of patients with secondary immune thrombocytopenia: retrospective EHR analysis.Ann Hematol. 2022 Jan;101(1):11-19. doi: 10.1007/s00277-021-04637-2. Epub 2021 Sep 10. Ann Hematol. 2022. PMID: 34505942 Free PMC article.
-
Eltrombopag in Immune Thrombocytopenia, Aplastic Anemia, and Myelodysplastic Syndrome: From Megakaryopoiesis to Immunomodulation.Drugs. 2019 Aug;79(12):1305-1319. doi: 10.1007/s40265-019-01159-0. Drugs. 2019. PMID: 31292909 Review.
References
-
- Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113:2386‐2393. - PubMed
-
- Fogarty PF. Chronic immune thrombocytopenia in adults: epidemiology and clinical presentation. Hematol Oncol Clin North Am. 2009;23:1213‐1221. - PubMed
-
- Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. The incidence of immune thrombocytopenic purpura in children and adults: a critical review of published reports. Am J Hematol. 2010;85:174‐180. - PubMed
-
- Kurata Y, Fujimura K, Kuwana M, Tomiyama Y, Murata M. Epidemiology of primary immune thrombocytopenia in children and adults in Japan: a population‐based study and literature review. Int J Hematol. 2011;93:329‐335. - PubMed
-
- British Committee for Standards in Haematology General Haematology Task Force . Guidelines for the investigation and management of idiopathic thrombocytopenic purpura in adults, children and in pregnancy. Br J Haematol. 2003;120:574‐596. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
