Sensitivity of MG-ADL for generalized weakness in myasthenia gravis
- PMID: 30417962
- PMCID: PMC6590478
- DOI: 10.1111/ene.13867
Sensitivity of MG-ADL for generalized weakness in myasthenia gravis
Abstract
Background and purpose: Myasthenia gravis activities of daily living (MG-ADL) is a commonly used questionnaire in MG trials. To investigate whether MG-ADL is equally sensitive to oculobulbar and generalized weakness, its correlation with the oculobulbar and generalized domain of the quantitative myasthenia gravis (QMG) score was analyzed (QMGob and QMGgen, respectively). To test whether the sensitivity of MG-ADL for generalized weakness could be improved, the additional value of ACTIVLIM on top of MG-ADL in the prediction QMGgen in was investigated.
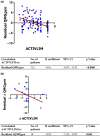
Methods: MG-ADL, QMG and ACTIVLIM, an ADL questionnaire focusing on generalized weakness, were analyzed in a prospective cohort of 112 MG patients. A generalized linear model was used to calculate the correlation of MG-ADL with QMGob and QMGgen and to assess the additional value of ACTIVLIM on top of MG-ADL for its correlation with QMGgen.
Results: MG-ADL had a higher correlation with QMGob than with QMGgen (B = 0.68, P < 0.001, and B = 0.38, P < 0.001, respectively). A similar trend was found for changes in the scores (B = 0.68, P = 0.132, and B = 0.39, P = 0.492, respectively). ACTIVLIM had a significant additional value on top of MG-ADL in the prediction of QMGgen, both cross-sectionally (B = -0.61, P < 0.001) and for changes within individual patients (B = -0.93, P = 0.041).
Conclusion: MG-ADL has a lower sensitivity for generalized weakness than for oculobulbar weakness. Adding questions on generalized weakness would improve the sensitivity of the MG-ADL for generalized weakness.
Keywords: myasthenia gravis; myasthenia gravis activities of daily living; neuromuscular disease; outcome measure; quantitative myasthenia gravis.
© 2018 The Authors European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
The authors declare no financial or other conflicts of interest.
Figures
Similar articles
-
Myasthenia Gravis Impairment Index: Sensitivity for Change in Generalized Muscle Weakness.J Neuromuscul Dis. 2020;7(3):297-300. doi: 10.3233/JND-200484. J Neuromuscul Dis. 2020. PMID: 32250313 Free PMC article.
-
Cigarette Smoking and Activities of Daily Living in Ocular Myasthenia Gravis.J Neuroophthalmol. 2016 Mar;36(1):37-40. doi: 10.1097/WNO.0000000000000306. J Neuroophthalmol. 2016. PMID: 26457691 Free PMC article.
-
Correlation of Quantitative Myasthenia Gravis and Myasthenia Gravis Activities of Daily Living scales in the MGTX study.Muscle Nerve. 2020 Aug;62(2):261-266. doi: 10.1002/mus.26910. Epub 2020 Jun 4. Muscle Nerve. 2020. PMID: 32369631 Free PMC article.
-
The myasthenia gravis--specific activities of daily living profile.Ann N Y Acad Sci. 2012 Dec;1274:114-9. doi: 10.1111/j.1749-6632.2012.06817.x. Ann N Y Acad Sci. 2012. PMID: 23252905 Review.
-
[Seronegative myasthenia gravis].Rev Neurol (Paris). 2004 Feb;160(2):159-62. doi: 10.1016/s0035-3787(04)70886-3. Rev Neurol (Paris). 2004. PMID: 15034472 Review. French.
Cited by
-
In-depth peripheral CD4+ T profile correlates with myasthenic crisis.Ann Clin Transl Neurol. 2021 Apr;8(4):749-762. doi: 10.1002/acn3.51312. Epub 2021 Feb 22. Ann Clin Transl Neurol. 2021. PMID: 33616296 Free PMC article.
-
Short-term outcome prediction for myasthenia gravis: an explainable machine learning model.Ther Adv Neurol Disord. 2023 Feb 24;16:17562864231154976. doi: 10.1177/17562864231154976. eCollection 2023. Ther Adv Neurol Disord. 2023. PMID: 36860354 Free PMC article.
-
Different Monoclonal Antibodies in Myasthenia Gravis: A Bayesian Network Meta-Analysis.Front Pharmacol. 2022 Jan 18;12:790834. doi: 10.3389/fphar.2021.790834. eCollection 2021. Front Pharmacol. 2022. PMID: 35115936 Free PMC article. Review.
-
Myasthenia Gravis Impairment Index: Sensitivity for Change in Generalized Muscle Weakness.J Neuromuscul Dis. 2020;7(3):297-300. doi: 10.3233/JND-200484. J Neuromuscul Dis. 2020. PMID: 32250313 Free PMC article.
-
Outcomes and characteristics of myasthenia gravis: A 10-year retrospective cross-sectional study at King Fahad Medical City.Neurosciences (Riyadh). 2022 Oct;27(4):237-243. doi: 10.17712/nsj.2022.4.20220038. Neurosciences (Riyadh). 2022. PMID: 36252965 Free PMC article.
References
-
- Rodolico C, Parisi D, Portaro S, et al Myasthenia gravis: unusual presentations and diagnostic pitfalls. J Neuromuscul Dis 2016; 3: 413–418. - PubMed
-
- Howard JF Jr, Utsugisawa K, Benatar M, et al Safety and efficacy of eculizumab in anti‐acetylcholine receptor antibody‐positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double‐blind, placebo‐controlled, multicentre study. Lancet Neurol 2017; 16: 976–986. - PubMed
-
- Beecher G, Anderson D, Siddiqi ZA. Subcutaneous immunoglobulin in myasthenia gravis exacerbation: a prospective, open‐label trial. Neurology 2017; 89: 1135–1141. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

