Regional Body Fat Changes and Metabolic Complications in Children With Dunnigan Lipodystrophy-Causing LMNA Variants
- PMID: 30418556
- PMCID: PMC6382455
- DOI: 10.1210/jc.2018-01922
Regional Body Fat Changes and Metabolic Complications in Children With Dunnigan Lipodystrophy-Causing LMNA Variants
Abstract
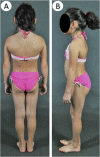
Context: Familial partial lipodystrophy, Dunnigan variety (FPLD2) is a rare autosomal-dominant disorder due to heterozygous missense lamin A/C (LMNA) mutations. Subjects with FPLD2 gradually lose fat from the upper and lower extremities but gain fat in the face and neck around puberty. However, the precise onset of body fat changes and metabolic complications during childhood remains unknown.
Objective: To compare metabolic parameters and regional body fat in children with FPLD2 with the sex- and age-matched controls from the National Health and Nutrition Examination Survey (NHANES) 2005 to 2010.
Methods: We measured fasting serum triglycerides, glucose, and skinfold thicknesses in all children (aged 1 to 18 years) harboring FPLD2-causing LMNA mutations and determined regional body fat by dual-energy X-ray absorptiometry in those aged ≥8 years.
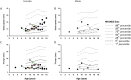
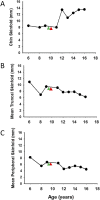
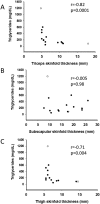
Results: Thirty-two affected females and 14 males participated. The lower limb fat in all affected females, except one, was below or equal to the first percentile and in two affected males was below the fifth percentile for NHANES. One female subject with FPLD2 followed from age 6 to 16 years revealed marked loss of extremity fat much before thelarche. Serum triglycerides were higher in females with FPLD2 aged 7 to 18 years compared with controls (median 208 vs 70 mg/dL; P < 0.0001) and showed inverse correlation with extremity skinfolds. Serum triglycerides in males with FPLD2 were not significantly different than controls.
Conclusions: The onset of fat loss from the extremities, especially in girls with FPLD2, occurs well before the onset of puberty. High serum triglycerides are seen in young females with FPLD2 with severe loss of fat from the extremities.
Copyright © 2019 Endocrine Society.
Figures


References
-
- Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350(12):1220–1234. - PubMed
-
- Peters JM, Barnes R, Bennett L, Gitomer WM, Bowcock AM, Garg A. Localization of the gene for familial partial lipodystrophy (Dunnigan variety) to chromosome 1q21-22. Nat Genet. 1998;18(3):292–295. - PubMed
-
- Cao H, Hegele RA. Nuclear lamin A/C R482Q mutation in canadian kindreds with Dunnigan-type familial partial lipodystrophy. Hum Mol Genet. 2000;9(1):109–112. - PubMed
-
- Shackleton S, Lloyd DJ, Jackson SN, Evans R, Niermeijer MF, Singh BM, Schmidt H, Brabant G, Kumar S, Durrington PN, Gregory S, O’Rahilly S, Trembath RC. LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat Genet. 2000;24(2):153–156. - PubMed
-
- Speckman RA, Garg A, Du F, Bennett L, Veile R, Arioglu E, Taylor SI, Lovett M, Bowcock AM. Mutational and haplotype analyses of families with familial partial lipodystrophy (Dunnigan variety) reveal recurrent missense mutations in the globular C-terminal domain of lamin A/C. Am J Hum Genet. 2000;66(4):1192–1198. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

