A Chronically Implantable Neural Coprocessor for Investigating the Treatment of Neurological Disorders
- PMID: 30418885
- PMCID: PMC6415546
- DOI: 10.1109/TBCAS.2018.2880148
A Chronically Implantable Neural Coprocessor for Investigating the Treatment of Neurological Disorders
Abstract
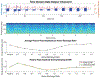
Developing new tools to better understand disorders of the nervous system, with a goal to more effectively treat them, is an active area of bioelectronic medicine research. Future tools must be flexible and configurable, given the evolving understanding of both neuromodulation mechanisms and how to configure a system for optimal clinical outcomes. We describe a system, the Summit RC+S "neural coprocessor," that attempts to bring the capability and flexibility of a microprocessor to a prosthesis embedded within the nervous system. This paper describes the updated system architecture for the Summit RC+S system, the five custom integrated circuits required for bi-directional neural interfacing, the supporting firmware/software ecosystem, and the verification and validation activities to prepare for human implantation. Emphasis is placed on design changes motivated by experience with the CE-marked Activa PC+S research tool; specifically, enhancement of sense-stim performance for improved bi-directional communication to the nervous system, implementation of rechargeable technology to extend device longevity, and application of MICS-band telemetry for algorithm development and data management. The technology was validated in a chronic treatment paradigm for canines with naturally occurring epilepsy, including free ambulation in the home environment, which represents a typical use case for future human protocols.
Figures





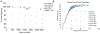



References
-
- Perestelo-Pérez L, et al. “Deep brain stimulation in Parkinson’s disease: meta-analysis of randomized controlled trials. J Neurol 2014; February 2. - PubMed
-
- Schuurman PR, et al. “A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor“ N Engl J Med 2000. February 17;342(7):461–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

