Effect of a Program Combining Transitional Care and Long-term Self-management Support on Outcomes of Hospitalized Patients With Chronic Obstructive Pulmonary Disease: A Randomized Clinical Trial
- PMID: 30419103
- PMCID: PMC6583083
- DOI: 10.1001/jama.2018.17933
Effect of a Program Combining Transitional Care and Long-term Self-management Support on Outcomes of Hospitalized Patients With Chronic Obstructive Pulmonary Disease: A Randomized Clinical Trial
Retraction in
-
Notice of Retraction. Aboumatar et al. Effect of a Program Combining Transitional Care and Long-term Self-management Support on Outcomes of Hospitalized Patients With Chronic Obstructive Pulmonary Disease: A Randomized Clinical Trial. JAMA. 2018;320(22):2335-2343.JAMA. 2019 Oct 8;322(14):1417-1418. doi: 10.1001/jama.2019.11954. JAMA. 2019. PMID: 31593277 No abstract available.
Abstract
Importance: Patients hospitalized for chronic obstructive pulmonary disease (COPD) exacerbations have high rehospitalization rates and reduced quality of life.
Objective: To evaluate a hospital-initiated program that combined transition and long-term self-management support for patients hospitalized due to COPD and their family caregivers.
Design, setting, and participants: This single-site randomized clinical trial was conducted in Baltimore, Maryland, with 240 participants. Participants were patients hospitalized due to COPD, randomized to intervention or usual care, and followed up for 6 months after hospital discharge. Enrollment occurred from March 2015 to May 2016; follow-up ended in December 2016.
Interventions: The intervention (n = 120) was a comprehensive 3-month program to help patients and their family caregivers with long-term self-management of COPD. It was delivered by COPD nurses (nurses with special training on supporting patients with COPD using standardized tools). Usual care (n = 120) included transition support for 30 days after discharge to ensure adherence to discharge plan and connection to outpatient care.
Main outcomes and measures: The primary outcome was number of COPD-related acute care events (hospitalizations and emergency department visits) per participant at 6 months. The co-primary outcome was change in participants' health-related quality of life measured by the St George's Respiratory Questionnaire (SGRQ) at 6 months after discharge (score, 0 [best] to 100 [worst]; 4-point difference is clinically meaningful).
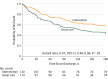
Results: Among 240 patients who were randomized (mean [SD] age, 64.9 [9.8] years; females, 61.7%), 203 (85%) completed the study. The mean (SD) baseline SGRQ score was 63.1 (19.9) in the intervention group and 62.6 (19.3) in the usual care group. The mean number of COPD-related acute care events per participant at 6 months was 0.72 (95% CI, 0.45-0.97) in the intervention group vs 1.40 (95% CI, 1.01-1.79) in the usual care group (difference, 0.68 [95% CI, 0.22 to 1.15]; P = .004). The mean change in participants' SGRQ total score at 6 months was -1.53 in the intervention and +5.44 in the usual care group (adjusted difference, -6.69 [95% CI, -12.97 to -0.40]; P = .04). During the study period, there were 15 deaths (intervention: 7; usual care: 8) and 337 hospitalizations (intervention: 135; usual care: 202).
Conclusions and relevance: In a single-site randomized clinical trial of patients hospitalized due to COPD, a 3-month program that combined transition and long-term self-management support resulted in significantly fewer COPD-related hospitalizations and emergency department visits and better health-related quality of life at 6 months after discharge. Further research is needed to evaluate this intervention in other settings.
Trial registration: ClinicalTrials.gov Identifier: NCT02036294.
Conflict of interest statement
Figures


Comment in
-
Intensive Intervention to Improve Outcomes for Patients With COPD.JAMA. 2018 Dec 11;320(22):2322-2324. doi: 10.1001/jama.2018.17508. JAMA. 2018. PMID: 30419098 No abstract available.
-
Self-medication as Part of Self-management Plans for Patients With COPD.JAMA. 2019 May 21;321(19):1937. doi: 10.1001/jama.2019.2048. JAMA. 2019. PMID: 31112252 No abstract available.
References
-
- Kochanek KD, Murphy SL, Xu JQ, Arias E. Mortality in the United States, 2016: NCHS Data Brief, No. 293. Hyattsville, MD: National Center for Health Statistics; 2017. - PubMed
-
- Brault MW, Hootman J, Helmick CG, Theis KA, Armour BS; Centers for Disease Control and Prevention (CDC) . Prevalence and most common causes of disability among adults: United States, 2005. MMWR Morb Mortal Wkly Rep. 2009;58(16):421-426. - PubMed

