Clinical characteristics of hepatic Arterioportal shunts associated with hepatocellular carcinoma
- PMID: 30419830
- PMCID: PMC6233279
- DOI: 10.1186/s12876-018-0899-3
Clinical characteristics of hepatic Arterioportal shunts associated with hepatocellular carcinoma
Abstract
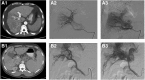
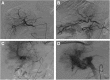
Background: Hepatic arterioportal shunt (A-P shunt) is defined as the direct blood flow established between hepatic artery and portal venous system; it is frequently observed in patients with hepatocellular carcinoma (HCC). Clinically, it is important to diagnose HCC associated A-P shunts, as it may impact the treatment strategy of the patients. In the present study, we described the imaging findings of the HCC associated A-P shunts and discussed the treatments strategy of such patients. From the findings, we also discussed the potential cause of A-P shunts.
Methods: Clinical data of HCC patients (n = 560), admitted to the hospital between April 2012 to April 2014, were reviewed. Hepatic angiography was used to examine the presence of A-P shunts. Of the 137 patients with A-P shunts, grading of the A-P shunts was performed, and statistical analysis of the different grades of A-P shunts and clinical characteristics was performed.
Results: The hepatic angiography confirmed that 99 patients had typical A-P shunts (Grade 1-3), and 38 patients had atypical A-P shunts. Embolization was the main strategy used to treat A-P shunts, in which liquid embolic agents appeared to provide a better treatment outcome. The correlation analysis showed that the grading of portal vein tumor thrombus was significantly associated with the grading of A-P shunt (p = < 0.001, Spearman correlation coefficient was 0.816 ± 0.043).
Conclusions: We characterized A-P shunts and proposed treatment strategy for treating HCC patients with various levels of A-P shunts. The findings supported the hypothesis that the formation of HCC associated A-P shunts was caused by tumor thrombus.
Keywords: Hepatic arterioportal shunts; Hepatocellular carcinoma; Portal vein tumor embolus; Transarterial chemoembolization.
Conflict of interest statement
Ethics approval and consent to participate
This study was carried out in accordance with the approved guidelines by the Ethics Committee of Shandong Tumor Hospital Affiliated to Shandong University (No. SDTHEC20171105) and patients have signed an informed consent.
Consent for publication
Not applicable.
Competing interests
All the authors have no conflict of interest for this study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

