Finding the right BCR-ABL1 tyrosine kinase inhibitor: a case report of successful treatment of a patient with chronic myeloid leukemia and a V299L mutation using nilotinib
- PMID: 30419862
- PMCID: PMC6233559
- DOI: 10.1186/s12885-018-5004-3
Finding the right BCR-ABL1 tyrosine kinase inhibitor: a case report of successful treatment of a patient with chronic myeloid leukemia and a V299L mutation using nilotinib
Abstract
Background: Chronic myeloid leukemia can be effectively treated with BCR-ABL1 tyrosine kinase inhibitors. However, BCR-ABL1 mutations can develop and cause secondary resistance to these inhibitors. For each of the available BCR-ABL1 inhibitors, certain mutations are known to be associated with resistance, although most mutations that confer resistance to one tyrosine kinase inhibitor remain sensitive to one or more of the other available inhibitors. For patients displaying poor response or loss of response to frontline treatment, the possibility that they have developed a new BCR-ABL1 mutation must be considered, and selection of a second-line treatment must consider the patient's mutational profile. Here we describe a case in which a patient developed a V299L mutation; although this mutation is known to be associated with resistance to dasatinib while remaining sensitive to nilotinib, limited information is currently available regarding the use of second-line nilotinib following development of a V299L mutation while receiving dasatinib.
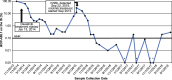
Case presentation: A 73-year-old man presenting with fatigue and drenching night sweats lasting for 2 weeks was diagnosed with chronic myeloid leukemia based on an analysis of a bone marrow biopsy and detection of the BCR-ABL1 fusion gene in peripheral blood. The patient initiated frontline treatment with dasatinib. A good treatment response was seen initially, with a complete hematologic response by month 2 of treatment. By month 20 however, BCR-ABL1 transcript levels rose markedly, and a mutational analysis revealed a BCR-ABL1 V299L mutation. Based on the identification of this specific mutation, the patient switched treatment to nilotinib; by month 18 of nilotinib treatment, the patient achieved a deeper reduction in BCR-ABL1 transcript levels than was seen with dasatinib. To date, in month 34 of treatment with nilotinib, the patient has shown good tolerance of the drug and has no clinical evidence of disease progression.
Conclusions: Our case report illustrates the benefit of having multiple drugs available to treat chronic myeloid leukemia, each with the ability to inhibit a distinct set of BCR-ABL1 mutations. This patient's case suggests that switching to nilotinib can be an effective treatment option for patients who develop a BCR-ABL1 V299L mutation while receiving dasatinib.
Keywords: Chronic myeloid leukemia; Dasatinib; Drug-resistant BCR-ABL mutations; Nilotinib; V299L.
Conflict of interest statement
Authors’ information
Not applicable.
Ethics approval and consent to participate
As this is a single case study that is reporting findings in retrospect, which had no intent of prospectively testing a hypothesis, Institutional Review Board approval was not sought. Verbal informed consent was obtained from the patient and was verified by two physicians who were present in person.
Consent for publication
Verbal informed consent has been obtained from the patient. Given that no standard consent form was available at the time consent was obtained, verbal informed consent from the patient was considered sufficient rather than written informed consent.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Melo JV. The diversity of BCR-ABL fusion proteins and their relationship to leukemia phenotype. Blood. 1996;88:2375–2384. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous