Meripilus giganteus ethanolic extract exhibits pro-apoptotic and anti-proliferative effects in leukemic cell lines
- PMID: 30419892
- PMCID: PMC6233556
- DOI: 10.1186/s12906-018-2366-7
Meripilus giganteus ethanolic extract exhibits pro-apoptotic and anti-proliferative effects in leukemic cell lines
Abstract
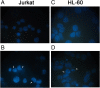
Background: The interest towards botanicals and plant extracts has strongly risen due to their numerous biological effects and ability to counteract chronic diseases development. Among these effects, chemoprevention which represents the possibility to counteract the cancerogenetic process is one of the most studied. The extracts of mushroom Meripilus giganteus (MG) (Phylum of Basidiomycota) showed to exert antimicrobic, antioxidant and antiproliferative effects. Therefore, since its effect in leukemic cell lines has not been previously evaluated, we studied its potential chemopreventive effect in Jurkat and HL-60 cell lines.
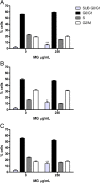
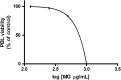
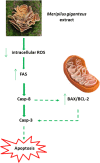
Methods: MG ethanolic extract was characterized for its antioxidant activity and scavenging effect against different radical species. Moreover, its phenolic profile was evaluated by HPLC-MS-MS analyses. Flow cytometry (FCM) analyses of Jurkat and HL-60 cells treated with MG extract (0-750 μg/mL) for 24-72 h- allowed to evaluate its cytotoxicity, pro-apoptotic and anti-proliferative effect. To better characterize MG pro-apoptotic mechanism ROS intracellular level and the gene expression level of FAS, BAX and BCL2 were also evaluated. Moreover, to assess MG extract selectivity towards cancer cells, its cytotoxicity was also evaluated in human peripheral blood lymphocytes (PBL).
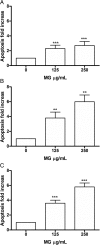
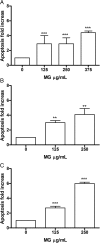
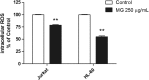
Results: MG extract induced apoptosis in Jurkat and HL-60 cells in a dose- and time- dependent manner by increasing BAX/BCL2 ratio, reducing ROS intracellular level and inducing FAS gene expression level. In fact, reduced ROS level is known to be related to the activation of apoptosis in leukemic cells by the involvement of death receptors. MG extract also induced cell-cycle arrest in HL-60 cells. Moreover, IC50 at 24 h treatment resulted 2 times higher in PBL than in leukemic cell lines.
Conclusions: Our data suggest that MG extract might be considered a promising and partially selective chemopreventive agent since it is able to modulate different mechanisms in transformed cells at concentrations lower than in non-transformed ones.
Keywords: Apoptosis; Chemoprevention; Cytotoxicity; Flow-cytometry; Leukemic cell lines; Meripilus giganteus.
Conflict of interest statement
Ethics approval and consent to participate
Authorization to the use of human blood samples (Buffy coat), for research purposes, has been obtained from AUSL Bologna IT, S. Orsola-Malpighi Hospital - PROT GEN n° 0051937, and written informed consent was obtained by AUSL Bologna IT, S. Orsola-Malpighi Hospital from donors for the use of their blood for scientific research purposes.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
Viscum articulatum Burm. f. aqueous extract exerts antiproliferative effect and induces cell cycle arrest and apoptosis in leukemia cells.J Ethnopharmacol. 2018 Jun 12;219:91-102. doi: 10.1016/j.jep.2018.03.005. Epub 2018 Mar 16. J Ethnopharmacol. 2018. PMID: 29555410
-
Castanea sativa Mill. bark extract exhibits chemopreventive properties triggering extrinsic apoptotic pathway in Jurkat cells.BMC Complement Altern Med. 2017 May 5;17(1):251. doi: 10.1186/s12906-017-1756-6. BMC Complement Altern Med. 2017. PMID: 28476162 Free PMC article.
-
Coriolus versicolor (Yunzhi) extract attenuates growth of human leukemia xenografts and induces apoptosis through the mitochondrial pathway.Oncol Rep. 2006 Sep;16(3):609-16. Oncol Rep. 2006. PMID: 16865263
-
The pro-apoptotic effect of a Terpene-rich Annona cherimola leaf extract on leukemic cell lines.BMC Complement Altern Med. 2019 Dec 12;19(1):365. doi: 10.1186/s12906-019-2768-1. BMC Complement Altern Med. 2019. PMID: 31830975 Free PMC article.
-
The Giant Polypore Mushroom Meripilus giganteus (Agaricomycetes): Promising Medicinal Applications (A Review).Int J Med Mushrooms. 2025;27(1):1-11. doi: 10.1615/IntJMedMushrooms.2024056428. Int J Med Mushrooms. 2025. PMID: 39717914 Review.
Cited by
-
Chemical profiles and pharmacological attributes of Apis cerana indica beehives using combined experimental and computer-aided studies.Heliyon. 2023 Mar 31;9(4):e15016. doi: 10.1016/j.heliyon.2023.e15016. eCollection 2023 Apr. Heliyon. 2023. PMID: 37089286 Free PMC article.
-
Ten Years of Research on Fucoidan and Cancer: Focus on Its Antiangiogenic and Antimetastatic Effects.Mar Drugs. 2023 May 18;21(5):307. doi: 10.3390/md21050307. Mar Drugs. 2023. PMID: 37233501 Free PMC article. Review.
-
Unveiling tribal treasures: myco-chemical characterization and pharmacological evaluation of an unexplored Russula pers. species.Antonie Van Leeuwenhoek. 2024 Oct 5;118(1):15. doi: 10.1007/s10482-024-02018-z. Antonie Van Leeuwenhoek. 2024. PMID: 39367931
-
Anxiolytic and Antidepressant Potential of Methanolic Extract of Neurada procumbens Linn. in Mice.Dose Response. 2023 Apr 13;21(2):15593258231169584. doi: 10.1177/15593258231169584. eCollection 2023 Apr-Jun. Dose Response. 2023. PMID: 37063345 Free PMC article.
-
Identification of Anti-Neuroinflammatory Bioactive Compounds in Essential Oils and Aqueous Distillation Residues Obtained from Commercial Varieties of Cannabis sativa L.Int J Mol Sci. 2023 Nov 22;24(23):16601. doi: 10.3390/ijms242316601. Int J Mol Sci. 2023. PMID: 38068924 Free PMC article.
References
-
- Tsai SY, Tsai HL, Mau JL. Antioxidant properties of Agaricus blazei, Agrocybe cylindracea, and Boletus edulis. LWT Food Sci Technol. 2007;40:1392–1402. doi: 10.1016/j.lwt.2006.10.001. - DOI
-
- Stamets P. Growing gourmet and medicinal mushrooms. New York: Ten Speed Press; 2000.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

