Sharing longitudinal, non-biological birth cohort data: a cross-sectional analysis of parent consent preferences
- PMID: 30419910
- PMCID: PMC6233367
- DOI: 10.1186/s12911-018-0683-x
Sharing longitudinal, non-biological birth cohort data: a cross-sectional analysis of parent consent preferences
Abstract
Background: Mandates abound to share publicly-funded research data for reuse, while data platforms continue to emerge to facilitate such reuse. Birth cohorts (BC) involve longitudinal designs, significant sample sizes and rich and deep datasets. Data sharing benefits include more analyses, greater research complexity, increased opportunities for collaboration, amplification of public contributions, and reduced respondent burdens. Sharing BC data involves significant challenges including consent, privacy, access policies, communication, and vulnerability of the child. Research on these issues is available for biological data, but these findings may not extend to BC data. We lack consensus on how best to approach these challenges in consent, privacy, communication and autonomy when sharing BC data. We require more stakeholder engagement to understand perspectives and generate consensus.
Methods: Parents participating in longitudinal birth cohorts completed a web-based survey investigating consent preferences for sharing their, and their child's, non-biological research data. Results from a previous qualitative inquiry informed survey development, and cognitive interviewing methods (n = 9) were used to improve the question quality and comprehension. Recruitment was via personalized email, with email and phone reminders during the 14-day window for survey completion.
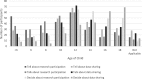
Results: Three hundred and forty-six of 569 parents completed the survey in September 2014 (60.8%). Participants preferred consent processes for data sharing in future independent research that were less-active (i.e. no consent or opt-out). Parents' consent preferences are associated with their communication preferences. Twenty percent (20.2%) of parents generally agreed that their child should provide consent to continue participating in research at age 12, while 25.6% felt decision-making on sharing non-biological research data should begin at age 18.
Conclusions: These finding reflect the parenting population's preference for less project-specific permission when research data is non-biological and de-identified and when governance practices are highly detailed and rigourous. Parents recognize that children should become involved in consent for secondary data use, but there is variability regarding when and how involvement occurs. These findings emphasize governance processes and participant notification rather than project-specific consent for secondary use of de-identified, non-biological data. Ultimately, parents prefer general consent processes for sharing de-identified, non-biological research data with ultimate involvement of the child.
Keywords: Consent; Data repository; Data sharing; Non-biological data; Parent; Pediatric.
Conflict of interest statement
Ethics approval and consent to participate
Ethics approval was obtained from the Conjoint Health Research Ethics Board (CHREB) at the University of Calgary prior to the start of the study (REB 13–1254). Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Organization for Economic Cooperation and Development. OECD principles and guidelines for access to research data from public funding. 2007. https://www.oecd.org/sti/sci-tech/38500813.pdf. Accessed 15 May 2017.
-
- Social Sciences and Humanities Research Council. Research data archiving policy. 2012. http://www.sshrc-crsh.gc.ca/about-au_sujet/policies-politiques/statement.... Accessed 13 Oct 2013.
-
- Canadian Institutes of Health Research. CIHR open access policy. 2013.http://www.cihr-irsc.gc.ca/e/46068.html. Accessed 13 Oct 2013.
-
- Canadian Institutes of Health Research. CIHR response and action plan - 2011 international review panel recommendations. Ottawa: Government of Canada; 2011.
-
- Medical Research Council. MRC policy and guidance on sharing of research data from population and patient studies. 2011 https://www.mrc.ac.uk/publications/browse/mrc-policy-and-guidance-on-sha.... Accessed 15 Oct 2013.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources