Impact of biobanks on research outcomes in rare diseases: a systematic review
- PMID: 30419920
- PMCID: PMC6233271
- DOI: 10.1186/s13023-018-0942-z
Impact of biobanks on research outcomes in rare diseases: a systematic review
Abstract
Background: Alleviating the burden of rare diseases requires research into new diagnostic and therapeutic strategies. We undertook a systematic review to identify and compare the impact of stand-alone registries, registries with biobanks, and rare disease biobanks on research outcomes in rare diseases.
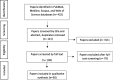
Methods: A systematic review and meta-aggregation was conducted using the preferred reporting items for systematic reviews and meta-analyses (the PRISMA statement). English language publications were sourced from PubMed, Medline, Scopus, and Web of Science. Original research papers that reported clinical, epidemiological, basic or translational research findings derived from data contained in stand-alone registries, registries with biobanks, and rare disease biobanks were considered. Articles selected for inclusion were assessed using the critical appraisal instruments by JBI-QARI. Each article was read in its entirety and findings were extracted using the online data extraction software from JBI-QARI.
Results: Thirty studies including 28 rare disease resources were included in the review. Of those, 14 registries were not associated to biobank infrastructure, 9 registries were associated with biobank infrastructure, and 6 were rare disease biobank resources. Stand-alone registries had the capacity to uncover the natural history of disease and contributed to evidence-based practice. When annexed to biobank infrastructure, registries could also identify and validate biomarkers, uncover novel genes, elucidate pathogenesis at the Omics level, and develop new therapeutic strategies. Rare disease biobanks in this review had similar capacity for biological investigations, but in addition, had far greater sample numbers and higher quality laboratory techniques for quality assurance processes.
Discussion: We examined the research outcomes of three specific populations: stand-alone registries, registries with biobanks, and stand-alone rare disease biobanks and demonstrated that there are key differences among these resources. These differences are a function of the resources' design, aims, and objectives, with each resource having a distinctive and important role in contributing to the body of knowledge for rare disease research. Whilst stand-alone registries had the capacity to uncover the natural history of disease, develop best practice, replace clinical trials, and improve patient outcomes, they were limited in their capacity to conduct basic research. The role of basic research in rare disease research is vital; scientists must first understand the pathways of disease before they can develop appropriate interventions. Rare disease biobanks, on the other hand (particularly larger biobanks), had the key infrastructure required to conduct basic research, making novel Omics discoveries, identify and validate biomarkers, uncover novel genes, and develop new therapeutic strategies. However, these stand-alone rare disease biobanks did not collect comprehensive data or impact on clinical observations like a rare disease registry. Rare disease research is important not only for rare diseases, but also for also common diseases. For example, research of low-density lipoprotein (LDL)-receptors in the rare disease known as familial hypercholesterolemia led to the discovery of statins, a drug therapy that is now used routinely to prevent heart disease.
Conclusions: Rare diseases are still under-researched worldwide. This review made the important observation that registries with biobanks had the function of both stand-alone registries (the capacity to collect comprehensive clinical and epidemiological data) and stand-alone rare disease biobanks (the ability to contribute to Omics research). We found registries with biobanks offer a unique, practical, cost-effective, and impactful solution for rare disease research. Linkage of stand-alone registries to rare disease biobanks will provide the appropriate resources required for the effective translation of basic research into clinical practice. Furthermore, facilitators such as collaboration, engagement, blended recruitment, pro-active marketing, broad consent, and "virtual biobank" online catalogues will, if utilised, add to the success of these resources. These important observations can serve to direct future rare diseases research efforts, ultimately improve patient outcomes and alleviate the significant burden associated with rare disease for clinicians, hospitals, society, and most importantly, the patients and their families.
Keywords: Biobank; Rare disease; Registries; Systematic review.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- European Commission. Rare diseases – what are they? (cited June 2018) https://ec.europa.eu/health/non_communicable_diseases/rare_diseases_en
-
- Kakkis E, O’Donovan M, Cox G, Hayes M, Goodsaid F, Tandon P, et al. Recommendations for the development of rare disease drugs using the accelerated approval pathway and for qualifying biomarkers as primary endpoints. Orphanet J Rare Dis. 2015;10(1):16. doi: 10.1186/s13023-014-0195-4. - DOI - PMC - PubMed
-
- Department of Health, Western Australia. WA Rare Diseases Strategic Framework 2015–2018. Perth: Office of Population Health Genomics, Public Health Division, Department of Health, Western Australia; 2015. (cited June 2018) http://ww2.health.wa.gov.au/Reports-and-publications/WA-Rare-Diseases-St...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

