PARPs in genome stability and signal transduction: implications for cancer therapy
- PMID: 30420415
- PMCID: PMC6299239
- DOI: 10.1042/BST20180418
PARPs in genome stability and signal transduction: implications for cancer therapy
Abstract
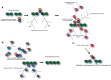
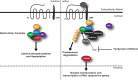
The poly(ADP-ribose) polymerase (PARP) superfamily of enzymes catalyses the ADP-ribosylation (ADPr) of target proteins by using nicotinamide adenine dinucleotide (NAD+) as a donor. ADPr reactions occur either in the form of attachment of a single ADP-ribose nucleotide unit on target proteins or in the form of ADP-ribose chains, with the latter called poly(ADP-ribosyl)ation. PARPs regulate many cellular processes, including the maintenance of genome stability and signal transduction. In this review, we focus on the PARP family members that possess the ability to modify proteins by poly(ADP-ribosyl)ation, namely PARP1, PARP2, Tankyrase-1, and Tankyrase-2. Here, we detail the cellular functions of PARP1 and PARP2 in the regulation of DNA damage response and describe the function of Tankyrases in Wnt-mediated signal transduction. Furthermore, we discuss how the understanding of these pathways has provided some major breakthroughs in the treatment of human cancer.
Keywords: DNA damage response; PARPs; adenosine diphosphate ribose; cancer; signalling.
© 2018 The Author(s).
Conflict of interest statement
The Authors declare that there are no competing interests associated with the manuscript.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

