The Long-Term Impact of Renin-Angiotensin System (RAS) Inhibition on Cardiorenal Outcomes (LIRICO): A Randomized, Controlled Trial
- PMID: 30420421
- PMCID: PMC6287867
- DOI: 10.1681/ASN.2018040443
The Long-Term Impact of Renin-Angiotensin System (RAS) Inhibition on Cardiorenal Outcomes (LIRICO): A Randomized, Controlled Trial
Abstract
Background: The comparative effectiveness of treatment with angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), or their combination in people with albuminuria and cardiovascular risk factors is unclear.
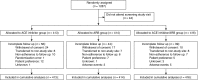
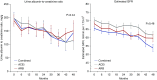
Methods: In a multicenter, randomized, open label, blinded end point trial, we evaluated the effectiveness on cardiovascular events of ACE or ARB monotherapy or combination therapy, targeting BP<130/80 in patients with moderate or severe albuminuria and diabetes or other cardiovascular risk factors. End points included a primary composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, and hospitalization for cardiovascular causes and a revised end point of all-cause mortality. Additional end points included ESRD, doubling of serum creatinine, albuminuria, eGFR, BP, and adverse events.
Results: Because of slow enrollment, the trial was modified and stopped 41% short of targeted enrollment of 2100 participants, corresponding to 35% power to detect a 25% reduced risk in the primary outcome. Our analysis included 1243 adults, with median follow-up of 2.7 years. Efficacy outcomes were similar between groups (ACE inhibitor versus ARB, ACE inhibitor versus combination, ARB versus combination) as were rates of serious adverse events. The rate of permanent discontinuation for ARB monotherapy (6.3%) was significantly lower than for ACE inhibitor monotherapy (15.7%) or combined therapy (18.3%).
Conclusions: Patients may tolerate ARB monotherapy better than ACE inhibitor monotherapy. However, data from this trial and similar trials, although as yet inconclusive, show no trend suggesting differences in mortality and renal outcomes with ACE inhibitors or ARBs as dual or monotherapy in patients with albuminuria and diabetes or other cardiovascular risk factors.
Keywords: albuminuria; clinical trial; diabetic nephropathy; end-stage renal disease; mortality; renin angiotensin system.
Copyright © 2018 by the American Society of Nephrology.
Figures


References
-
- Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, et al. .: ONTARGET Investigators : Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 358: 1547–1559, 2008 - PubMed
-
- de Boer IH, Bangalore S, Benetos A, Davis AM, Michos ED, Muntner P, et al. .: Diabetes and hypertension: A position statement by the American Diabetes Association. Diabetes Care 40: 1273–1284, 2017 - PubMed
-
- Barnett AH, Bain SC, Bouter P, Karlberg B, Madsbad S, Jervell J, et al. .: Diabetics Exposed to Telmisartan and Enalapril Study Group : Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med 351: 1952–1961, 2004 - PubMed
-
- Cheung R, Lewanczuk RZ, Rodger NW, Huff MW, Oddou-Stock P, Botteri F, et al. .: The effect of valsartan and captopril on lipid parameters in patients with type II diabetes mellitus and nephropathy. Int J Clin Pract 53: 584–592, 1999 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

