The rhomboid protease GlpG has weak interaction energies in its active site hydrogen bond network
- PMID: 30420443
- PMCID: PMC6400518
- DOI: 10.1085/jgp.201812047
The rhomboid protease GlpG has weak interaction energies in its active site hydrogen bond network
Abstract
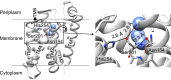
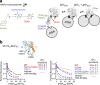
Intramembrane rhomboid proteases are of particular interest because of their function to hydrolyze a peptide bond of a substrate buried in the membrane. Crystal structures of the bacterial rhomboid protease GlpG have revealed a catalytic dyad (Ser201-His254) and oxyanion hole (His150/Asn154/the backbone amide of Ser201) surrounded by the protein matrix and contacting a narrow water channel. Although multiple crystal structures have been solved, the catalytic mechanism of GlpG is not completely understood. Because it is a serine protease, hydrogen bonding interactions between the active site residues are thought to play a critical role in the catalytic cycle. Here, we dissect the interaction energies among the active site residues His254, Ser201, and Asn154 of Escherichia coli GlpG, which form a hydrogen bonding network. We combine double mutant cycle analysis with stability measurements using steric trapping. In mild detergent, the active site residues are weakly coupled with interaction energies (ΔΔG Inter) of ‒1.4 kcal/mol between His254 and Ser201 and ‒0.2 kcal/mol between Ser201 and Asn154. Further, by analyzing the propagation of single mutations of the active site residues, we find that these residues are important not only for function but also for the folding cooperativity of GlpG. The weak interaction between Ser and His in the catalytic dyad may partly explain the unusually slow proteolysis by GlpG compared with other canonical serine proteases. Our result suggests that the weak hydrogen bonds in the active site are sufficient to carry out the proteolytic function of rhomboid proteases.
© 2019 Hong and Gaffney.
Figures


Comment in
-
Rhomboids make do with a weak hydrogen bond.J Gen Physiol. 2019 Mar 4;151(3):274. doi: 10.1085/jgp.201912334. Epub 2019 Feb 12. J Gen Physiol. 2019. PMID: 30755458 Free PMC article.
Similar articles
-
Substrate binding and specificity of rhomboid intramembrane protease revealed by substrate-peptide complex structures.EMBO J. 2014 Oct 16;33(20):2408-21. doi: 10.15252/embj.201489367. Epub 2014 Sep 12. EMBO J. 2014. PMID: 25216680 Free PMC article.
-
How does the exosite of rhomboid protease affect substrate processing and inhibition?Protein Sci. 2017 Dec;26(12):2355-2366. doi: 10.1002/pro.3294. Epub 2017 Oct 24. Protein Sci. 2017. PMID: 28884847 Free PMC article.
-
An internal water-retention site in the rhomboid intramembrane protease GlpG ensures catalytic efficiency.Structure. 2012 Jul 3;20(7):1255-63. doi: 10.1016/j.str.2012.04.022. Epub 2012 Jun 14. Structure. 2012. PMID: 22705210 Free PMC article.
-
Untangling structure-function relationships in the rhomboid family of intramembrane proteases.Biochim Biophys Acta. 2013 Dec;1828(12):2862-72. doi: 10.1016/j.bbamem.2013.05.003. Biochim Biophys Acta. 2013. PMID: 24099005 Review.
-
Structure and mechanism of rhomboid protease.J Biol Chem. 2013 May 31;288(22):15430-6. doi: 10.1074/jbc.R112.422378. Epub 2013 Apr 12. J Biol Chem. 2013. PMID: 23585569 Free PMC article. Review.
Cited by
-
Steric trapping strategy for studying the folding of helical membrane proteins.Methods. 2024 May;225:1-12. doi: 10.1016/j.ymeth.2024.02.007. Epub 2024 Feb 29. Methods. 2024. PMID: 38428472 Free PMC article. Review.
-
Rhomboids make do with a weak hydrogen bond.J Gen Physiol. 2019 Mar 4;151(3):274. doi: 10.1085/jgp.201912334. Epub 2019 Feb 12. J Gen Physiol. 2019. PMID: 30755458 Free PMC article.
-
Interpreting the molecular mechanisms of disease variants in human transmembrane proteins.Biophys J. 2023 Jun 6;122(11):2176-2191. doi: 10.1016/j.bpj.2022.12.031. Epub 2023 Jan 3. Biophys J. 2023. PMID: 36600598 Free PMC article.
-
Membrane proteins enter the fold.Curr Opin Struct Biol. 2021 Aug;69:124-130. doi: 10.1016/j.sbi.2021.03.006. Epub 2021 May 8. Curr Opin Struct Biol. 2021. PMID: 33975156 Free PMC article. Review.
-
Maintaining the Integral Membrane Proteome: Revisiting the Functional Repertoire of Integral Membrane Proteases.Chembiochem. 2025 May 5;26(9):e202500048. doi: 10.1002/cbic.202500048. Epub 2025 Mar 18. Chembiochem. 2025. PMID: 40056010 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

