E3611-A Randomized Phase II Study of Ipilimumab at 3 or 10 mg/kg Alone or in Combination with High-Dose Interferon-α2b in Advanced Melanoma
- PMID: 30420448
- PMCID: PMC6335150
- DOI: 10.1158/1078-0432.CCR-18-2258
E3611-A Randomized Phase II Study of Ipilimumab at 3 or 10 mg/kg Alone or in Combination with High-Dose Interferon-α2b in Advanced Melanoma
Abstract
Purpose: Interferon-α favors a Th1 shift in immunity, and combining with ipilimumab (ipi) at 3 or 10 mg/kg may downregulate CTLA4-mediated suppressive effects, leading to more durable antitumor immune responses. A study of tremelimumab and high-dose interferon-α (HDI) showed promising efficacy, supporting this hypothesis.
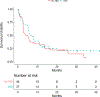
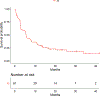
Patients and methods: E3611 followed a 2-by-2 factorial design (A: ipi10+HDI; B: ipi10; C: ipi3+HDI; D: ipi3) to evaluate (i) no HDI versus HDI (across ipilimumab doses) and (ii) ipi3 versus ipi10 (across HDI status). We hypothesized that median progression-free survival (PFS) would improve from 3 to 6 months with HDI versus no HDI and with ipi10 versus ipi3.
Results: For eligible and treated patients (N = 81) at a median follow-up time of 29.8 months, median PFS was 4.4 months [95% confidence interval (CI), 2.7-8.2] when ipilimumab was used alone and 7.5 months (95% CI, 5.1-11.0) when HDI was added. Median PFS was 3.8 months (95% CI, 2.6-7.5) with 3 mg/kg ipilimumab and 6.5 months (95% CI, 5.1-13.5) with 10 mg/kg. By study arm, median PFS was 8.0 months (95% CI, 2.8-20.2) in arm A, 6.2 months (95% CI, 2.7-25.7) in B, 5.7 months (95% CI, 1.5-11.1) in C, and 2.8 months (95% CI, 2.6-5.7) in D. The differences in PFS and overall survival (OS) did not reach statistical significance. Adverse events were consistent with the known profiles of ipilimumab and HDI and significantly higher with HDI and ipi10.
Conclusions: Although PFS was increased, the differences resulting from adding interferon-α or a higher dose of ipilimumab did not reach statistical significance and do not outweigh the added toxicity risks.
©2018 American Association for Cancer Research.
Conflict of interest statement
Conflicts of Interest:
The rest of the authors declare no potential conflicts of interest relevant to the study.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

