Tailoring of Proteostasis Networks with Heat Shock Factors
- PMID: 30420555
- PMCID: PMC6442201
- DOI: 10.1101/cshperspect.a034066
Tailoring of Proteostasis Networks with Heat Shock Factors
Abstract
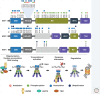
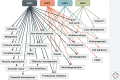
Heat shock factors (HSFs) are the main transcriptional regulators of the heat shock response and indispensable for maintaining cellular proteostasis. HSFs mediate their protective functions through diverse genetic programs, which are composed of genes encoding molecular chaperones and other genes crucial for cell survival. The mechanisms that are used to tailor HSF-driven proteostasis networks are not yet completely understood, but they likely comprise from distinct combinations of both genetic and proteomic determinants. In this review, we highlight the versatile HSF-mediated cellular functions that extend from cellular stress responses to various physiological and pathological processes, and we underline the key advancements that have been achieved in the field of HSF research during the last decade.
Copyright © 2019 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
