Vibrio cholerae motility exerts drag force to impede attack by the bacterial predator Bdellovibrio bacteriovorus
- PMID: 30420597
- PMCID: PMC6232129
- DOI: 10.1038/s41467-018-07245-3
Vibrio cholerae motility exerts drag force to impede attack by the bacterial predator Bdellovibrio bacteriovorus
Abstract
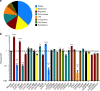
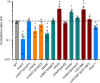
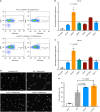
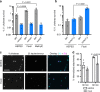
The bacterial predator Bdellovibrio bacteriovorus is evolved to attack and kill other bacteria, including the human intestinal pathogen Vibrio cholerae. Although B. bacteriovorus exhibit a broad prey range, little is known about the genetic determinants of prey resistance and sensitivity. Here we perform a genetic screen on V. cholerae and identify five pathways contributing to predation susceptibility. We find that the essential virulence regulators ToxR/S increase susceptibility to predation, as mutants of these genes are more resistant to predation. We observe by flow cytometry that lipopolysaccharide is a critical defense, as mutants lacking O-antigen are rapidly attacked by predatory B. bacteriovorus. Using polymer solutions to alter media viscosity, we find that when B. bacteriovorus attacks motile V. cholerae, increased drag forces slow its ability to prey. These results provide insights into key prey resistance mechanisms, and may be useful in the application of B. bacteriovorus in treating infections.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Fenton AK, Kanna M, Woods RD, Aizawa SI, Sockett RE. Shadowing the actions of a predator: backlit fluorescent microscopy reveals synchronous nonbinary septation of predatory Bdellovibrio inside prey and exit through discrete bdelloplast pores. J. Bacteriol. 2010;192:6329–6335. doi: 10.1128/JB.00914-10. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

