Human adipose glycerol flux is regulated by a pH gate in AQP10
- PMID: 30420639
- PMCID: PMC6232157
- DOI: 10.1038/s41467-018-07176-z
Human adipose glycerol flux is regulated by a pH gate in AQP10
Abstract
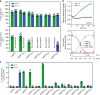
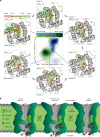
Obesity is a major threat to global health and metabolically associated with glycerol homeostasis. Here we demonstrate that in human adipocytes, the decreased pH observed during lipolysis (fat burning) correlates with increased glycerol release and stimulation of aquaglyceroporin AQP10. The crystal structure of human AQP10 determined at 2.3 Å resolution unveils the molecular basis for pH modulation-an exceptionally wide selectivity (ar/R) filter and a unique cytoplasmic gate. Structural and functional (in vitro and in vivo) analyses disclose a glycerol-specific pH-dependence and pinpoint pore-lining His80 as the pH-sensor. Molecular dynamics simulations indicate how gate opening is achieved. These findings unravel a unique type of aquaporin regulation important for controlling body fat mass. Thus, targeting the cytoplasmic gate to induce constitutive glycerol secretion may offer an attractive option for treating obesity and related complications.
Conflict of interest statement
S.K., M.S., C.H.N., and P.A.P. have interests in Aquaporin A/S. The remaining authors declare no competing interests.
Figures


References
-
- Meisner H, Tenney K. pH as an indicator of free fatty acid release from adipocytes. J. Lipid Res. 1977;18:774–776. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

