Near-infrared STED nanoscopy with an engineered bacterial phytochrome
- PMID: 30420676
- PMCID: PMC6232180
- DOI: 10.1038/s41467-018-07246-2
Near-infrared STED nanoscopy with an engineered bacterial phytochrome
Abstract
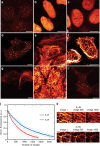
The near infrared (NIR) optical window between the cutoff for hemoglobin absorption at 650 nm and the onset of increased water absorption at 900 nm is an attractive, yet largely unexplored, spectral regime for diffraction-unlimited super-resolution fluorescence microscopy (nanoscopy). We developed the NIR fluorescent protein SNIFP, a bright and photostable bacteriophytochrome, and demonstrate its use as a fusion tag in live-cell microscopy and STED nanoscopy. We further demonstrate dual color red-confocal/NIR-STED imaging by co-expressing SNIFP with a conventional red fluorescent protein.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

