Identification of DKK-1 as a novel mediator of statin effects in human endothelial cells
- PMID: 30420710
- PMCID: PMC6232108
- DOI: 10.1038/s41598-018-35119-7
Identification of DKK-1 as a novel mediator of statin effects in human endothelial cells
Abstract
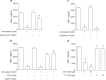
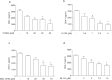
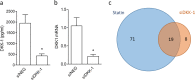
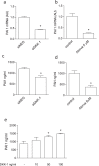
This study shows that DKK-1, a member of the Dickkopf family and a regulator of the Wnt pathways, represents a novel target of statins which, through the inhibition of HMG-CoA reductase and of non-steroidal isoprenoid intermediates, exert extra-beneficial effect in preventing atherosclerosis beyond their effect on the lipid profile. We found that atorvastatin downregulates DKK-1 protein (-88.3 ± 4.1%) and mRNA expression (-90 ± 4.2%) through the inhibition of Cdc42, Rho and Rac geranylgeranylated proteins. Further, a combined approach based on the integration of label-free quantitative mass spectrometry based-proteomics and gene silencing allowed us to demonstrate that DKK-1 itself mediates, at least in part, statin effects on human endothelial cells. Indeed, DKK-1 is responsible for the regulation of the 21% of the statin-modulated proteins, which include, among others, clusterin/apoJ, plasminogen activator inhibitor type 1 (PAI-1), myristoylated alanine-rich C-kinase substrate (MARCKS), and pentraxin 3 (PTX3). The Gene Ontology enrichment annotation revealed that DKK-1 is also a potential mediator of the extracellular matrix organization, platelet activation and response to wounding processes induced by statin. Finally, we found that plasma level of DKK-1 from cholesterol-fed rabbits treated with atorvastatin (2.5 mg/kg/day for 8 weeks) was lower (-42 ± 23%) than that of control animals. Thus, DKK-1 is not only a target of statin but it directly regulates the expression of molecules involved in a plethora of biological functions, thus expanding its role, which has been so far restricted mainly to cancer.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Atorvastatin reduces long pentraxin 3 expression in vascular cells by inhibiting protein geranylgeranylation.Vascul Pharmacol. 2015 Apr-Jun;67-69:38-47. doi: 10.1016/j.vph.2014.11.008. Epub 2015 Apr 4. Vascul Pharmacol. 2015. PMID: 25849951
-
Proteomic analysis of endothelial cell secretome: a means of studying the pleiotropic effects of Hmg-CoA reductase inhibitors.J Proteomics. 2013 Jan 14;78:346-61. doi: 10.1016/j.jprot.2012.10.003. Epub 2012 Oct 17. J Proteomics. 2013. PMID: 23085226
-
Dickkopf-1 is regulated by the mevalonate pathway in breast cancer.Breast Cancer Res. 2014 Feb 14;16(1):R20. doi: 10.1186/bcr3616. Breast Cancer Res. 2014. PMID: 24528599 Free PMC article.
-
Dkk (Dickkopf) Proteins.Arterioscler Thromb Vasc Biol. 2019 Jul;39(7):1330-1342. doi: 10.1161/ATVBAHA.119.312612. Epub 2019 May 16. Arterioscler Thromb Vasc Biol. 2019. PMID: 31092014 Review.
-
The antithrombotic effects of statins.Annu Rev Med. 2014;65:433-45. doi: 10.1146/annurev-med-051812-145304. Annu Rev Med. 2014. PMID: 24422578 Review.
Cited by
-
Prenylcysteine oxidase 1, an emerging player in atherosclerosis.Commun Biol. 2021 Sep 21;4(1):1109. doi: 10.1038/s42003-021-02630-z. Commun Biol. 2021. PMID: 34548610 Free PMC article.
-
RNA Sequencing Reveals Beneficial Effects of Atorvastatin on Endothelial Cells in Acute Kawasaki Disease.J Am Heart Assoc. 2022 Jul 19;11(14):e025408. doi: 10.1161/JAHA.122.025408. Epub 2022 Jul 15. J Am Heart Assoc. 2022. PMID: 35861833 Free PMC article.
-
Multiplexed MRM-based proteomics for identification of circulating proteins as biomarkers of cardiovascular damage progression associated with diabetes mellitus.Cardiovasc Diabetol. 2024 Jan 20;23(1):36. doi: 10.1186/s12933-024-02125-1. Cardiovasc Diabetol. 2024. PMID: 38245742 Free PMC article.
-
Comparison of Maternal Serum Levels and Placental mRNA Levels of Dickkopf-1 in Preeclamptic and Normal Pregnant Women at Delivery.Geburtshilfe Frauenheilkd. 2021 Nov 4;81(11):1247-1255. doi: 10.1055/a-1557-1234. eCollection 2021 Nov. Geburtshilfe Frauenheilkd. 2021. PMID: 34754274 Free PMC article.
-
Prenylcysteine Oxidase 1 Is a Key Regulator of Adipogenesis.Antioxidants (Basel). 2023 Feb 21;12(3):542. doi: 10.3390/antiox12030542. Antioxidants (Basel). 2023. PMID: 36978789 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

