Venetoclax with azacitidine disrupts energy metabolism and targets leukemia stem cells in patients with acute myeloid leukemia
- PMID: 30420752
- PMCID: PMC7001730
- DOI: 10.1038/s41591-018-0233-1
Venetoclax with azacitidine disrupts energy metabolism and targets leukemia stem cells in patients with acute myeloid leukemia
Abstract
Acute myeloid leukemia (AML) is the most common acute leukemia in adults. Leukemia stem cells (LSCs) drive the initiation and perpetuation of AML, are quantifiably associated with worse clinical outcomes, and often persist after conventional chemotherapy resulting in relapse1-5. In this report, we show that treatment of older patients with AML with the B cell lymphoma 2 (BCL-2) inhibitor venetoclax in combination with azacitidine results in deep and durable remissions and is superior to conventional treatments. We hypothesized that these promising clinical results were due to targeting LSCs. Analysis of LSCs from patients undergoing treatment with venetoclax + azacitidine showed disruption of the tricarboxylic acid (TCA) cycle manifested by decreased α-ketoglutarate and increased succinate levels, suggesting inhibition of electron transport chain complex II. In vitro modeling confirmed inhibition of complex II via reduced glutathionylation of succinate dehydrogenase. These metabolic perturbations suppress oxidative phosphorylation (OXPHOS), which efficiently and selectively targets LSCs. Our findings show for the first time that a therapeutic intervention can eradicate LSCs in patients with AML by disrupting the metabolic machinery driving energy metabolism, resulting in promising clinical activity in a patient population with historically poor outcomes.
Conflict of interest statement
Competing interests Statement
The authors declare no competing interests.
Figures
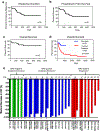


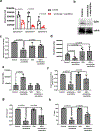
Comment in
-
Venetolax with Azacitidine Drains Fuel from AML Stem Cells.Cell Stem Cell. 2019 Jan 3;24(1):7-8. doi: 10.1016/j.stem.2018.12.005. Cell Stem Cell. 2019. PMID: 30609400
References
-
- Bonnet D & Dick JE Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 3, 730–737 (1997). - PubMed
-
- van Rhenen A, et al. High stem cell frequency in acute myeloid leukemia at diagnosis predicts high minimal residual disease and poor survival. Clin Cancer Res 11, 6520–6527 (2005). - PubMed
-
- Lapidot T, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367, 645–648 (1994). - PubMed
-
- Jordan CT, Guzman ML & Noble M Cancer stem cells. N Engl J Med 355, 1253–1261 (2006). - PubMed
References (Methods only)
-
- DiNardo CD, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol 19, 216–228 (2018). - PubMed
-
- Slovak ML, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood 96, 4075–4083 (2000). - PubMed
-
- Cheson BD, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol 21, 4642–4649 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

