Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade
- PMID: 30420753
- PMCID: PMC6324991
- DOI: 10.1038/s41591-018-0221-5
Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade
Abstract
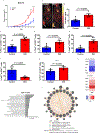
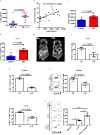
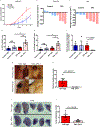
The recent successes of immunotherapy have shifted the paradigm in cancer treatment, but because only a percentage of patients are responsive to immunotherapy, it is imperative to identify factors impacting outcome. Obesity is reaching pandemic proportions and is a major risk factor for certain malignancies, but the impact of obesity on immune responses, in general and in cancer immunotherapy, is poorly understood. Here, we demonstrate, across multiple species and tumor models, that obesity results in increased immune aging, tumor progression and PD-1-mediated T cell dysfunction which is driven, at least in part, by leptin. However, obesity is also associated with increased efficacy of PD-1/PD-L1 blockade in both tumor-bearing mice and clinical cancer patients. These findings advance our understanding of obesity-induced immune dysfunction and its consequences in cancer and highlight obesity as a biomarker for some cancer immunotherapies. These data indicate a paradoxical impact of obesity on cancer. There is heightened immune dysfunction and tumor progression but also greater anti-tumor efficacy and survival after checkpoint blockade which directly targets some of the pathways activated in obesity.
Conflict of interest statement
Competing Interests Statement
The authors have no competing interests to declare.
Figures



Comment in
-
The 'obesity paradox' in action with cancer immunotherapy.Nat Rev Endocrinol. 2019 Mar;15(3):132-133. doi: 10.1038/s41574-019-0161-2. Nat Rev Endocrinol. 2019. PMID: 30670818 No abstract available.
-
Is obesity a disease?-the evolving concepts, cancer paradox and association with improved cancer immunotherapy efficacy.Hepatobiliary Surg Nutr. 2020 Apr;9(2):247-249. doi: 10.21037/hbsn.2019.11.35. Hepatobiliary Surg Nutr. 2020. PMID: 32355693 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA093373/CA/NCI NIH HHS/United States
- U24 DK092993/DK/NIDDK NIH HHS/United States
- T32 CA009138/CA/NCI NIH HHS/United States
- R01 CA195904/CA/NCI NIH HHS/United States
- R24 HL085794/HL/NHLBI NIH HHS/United States
- R01 CA214048/CA/NCI NIH HHS/United States
- R01 HL056067/HL/NHLBI NIH HHS/United States
- R01 CA095572/CA/NCI NIH HHS/United States
- P01 CA065493/CA/NCI NIH HHS/United States
- S10 OD018223/OD/NIH HHS/United States
- R01 HL118979/HL/NHLBI NIH HHS/United States
- P51 OD011107/OD/NIH HHS/United States
- R37 AI034495/AI/NIAID NIH HHS/United States
- P30 CA225520/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

