Influence of Strategic Cortical Infarctions on Pupillary Function
- PMID: 30420833
- PMCID: PMC6215832
- DOI: 10.3389/fneur.2018.00916
Influence of Strategic Cortical Infarctions on Pupillary Function
Abstract
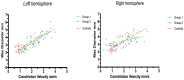
Objective: Cortical activity, including cognitive and emotional processes, may influence pupillary function. The exact pathways and the site of cortical pupillary innervation remain elusive, however. We investigated the effects of select cortical strokes, i.e. ischemic infarcts affecting the insular cortex and prefrontal eye field, on pupillary function. Methods: Seventy-four patients with acute ischemic stroke, consecutively admitted to our institution from March to July 2018, were assessed 24 h after endovascular recanalization therapy (i.e., day 2 after the stroke), using automated pupillometry. Stroke location and volume and clinical severity (estimated by the Alberta Stroke Program Early CT Score and National Institute of Health Stroke Scale) were recorded. We excluded patients with posterior circulation stroke, intracranial pathology other than ischemic stroke, midline shift on computed tomography exceeding 5 millimeters or a history of eye disease. Pupillometry data from 25 neurologically normal patients with acute myocardial infarction were acquired for control. Results: Fifty stroke patients after thrombectomy were included for analysis. Twenty-five patients (50%) had insular cortex or prefrontal eye field involvement (group 1, strategic infarcts); 25 patients had infarcts located in other cerebral areas (group 2, other infarcts). The pupillary light reflex, as measured by constriction velocity and maximal/minimal pupillary diameters, was within physiological limits in all patients, including controls. However, while pupillary size and constriction velocities were correlated in all subjects, the correlation of size and dilatation velocity was absent in right-hemispheric infarcts (left hemisphere infarcts, group 1 (r 2 = 0.15, p = 0.04), group 2 (r 2 = 0.41, p = 0.0007); right hemisphere infarcts, group 1 (r 2 = 0.008, p = 0.69); group 2 (r 2 = 0.12, p = 0.08); controls (r 2 = 0.29, p ≤ 0.0001). Conclusions: Cortical infarcts of the prefrontal eye field or insula do not impair the pupillary light reflex in humans. However, subtle changes may occur when the pupils dilate back to baseline, probably due to autonomic dysfunction. Replication is needed to explore the possible influence of hemispheric lateralization. We suggest that endovascular therapy for acute ischemic stroke may serve as a clinical research model for the study of acquired cortical lesions in humans.
Keywords: endovascular stroke therapy; insula; mechanical thrombectomy; prefrontal eye field; pupillary light reflex; pupillometry; pupils; stroke.
Figures




References
LinkOut - more resources
Full Text Sources

