Key Components for Antibiotic Dose Optimization of Sepsis in Neonates and Infants
- PMID: 30420947
- PMCID: PMC6215831
- DOI: 10.3389/fped.2018.00325
Key Components for Antibiotic Dose Optimization of Sepsis in Neonates and Infants
Abstract
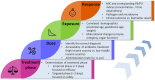
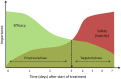
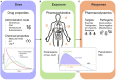
Sepsis in neonates and infants remains a major cause of death despite a decline in child mortality and morbidity over the last decades. A key factor in further reducing poor clinical outcomes is the optimal use of antibiotics in sepsis management. Developmental changes such as maturation of organ function and capacity of drug metabolizing enzymes can affect the pharmacokinetic profile and therefore the antibiotic exposure and response in neonates and infants. Optimal antibiotic treatment of sepsis in neonates and young infants is dependent on several key components such as the determination of treatment phase, the administered dose and the resulted drug exposure and microbiological response. During the initial phase of suspected sepsis, the primary focus of empirical treatment is to assure efficacy. Once bacterial infection as the cause of sepsis is confirmed the focus shifts toward a targeted treatment, ensuring an optimal balance between efficacy and safety. Interpretation of antibiotic exposure and microbiological response in neonates and infants is multifaceted. The response or treatment effect can be determined by the microbiological parameters (MIC) together with the characteristics of the pathogen (time- or concentration dependent). The antibiotic response is influenced by the properties of the causative pathogen and the unique characteristics of the vulnerable patient population such as reduced humoral response or reduced skin barrier function. Therapeutic drug monitoring (TDM) of antibiotics may be used to increase effectiveness while maximizing safety and minimizing the toxicity, but requires expertise in different fields and requires collaborations between physicians, lab technicians, and quantitative clinical pharmacologists. Understanding these clinical, pharmacological, and microbiological components and their underlying relationship can provide a scientific basic for proper antibiotic use and reduction of antibiotic resistance in neonates and infants. This highlights the necessity of a close multidisciplinary collaboration between physicians, pharmacists, clinical pharmacologists and microbiologist to assure the optimal utilization of antibiotics in neonates and young infants.
Keywords: antibiotics; empirical phase; exposure; neonates; sepsis; targeted phase.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

