Inherited Disorders of Iron Overload
- PMID: 30420953
- PMCID: PMC6215844
- DOI: 10.3389/fnut.2018.00103
Inherited Disorders of Iron Overload
Abstract
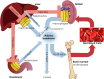
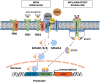
Dietary iron absorption and systemic iron traffic are tightly controlled by hepcidin, a liver-derived peptide hormone. Hepcidin inhibits iron entry into plasma by binding to and inactivating the iron exporter ferroportin in target cells, such as duodenal enterocytes and tissue macrophages. Hepcidin is induced in response to increased body iron stores to inhibit further iron absorption and prevent iron overload. The mechanism involves the BMP/SMAD signaling pathway, which triggers transcriptional hepcidin induction. Inactivating mutations in components of this pathway cause hepcidin deficiency, which allows inappropriately increased iron absorption and efflux into the bloodstream. This leads to hereditary hemochromatosis (HH), a genetically heterogenous autosomal recessive disorder of iron metabolism characterized by gradual buildup of unshielded non-transferrin bound iron (NTBI) in plasma and excessive iron deposition in tissue parenchymal cells. The predominant HH form is linked to mutations in the HFE gene and constitutes the most frequent genetic disorder in Caucasians. Other, more severe and rare variants are caused by inactivating mutations in HJV (hemojuvelin), HAMP (hepcidin) or TFR2 (transferrin receptor 2). Mutations in SLC40A1 (ferroportin) that cause hepcidin resistance recapitulate the biochemical phenotype of HH. However, ferroportin-related hemochromatosis is transmitted in an autosomal dominant manner. Loss-of-function ferroportin mutations lead to ferroportin disease, characterized by iron overload in macrophages and low transferrin saturation. Aceruloplasminemia and atransferrinemia are further inherited disorders of iron overload caused by deficiency in ceruloplasmin or transferrin, the plasma ferroxidase and iron carrier, respectively.
Keywords: HFE; aceruloplasminemia; ferroportin; hemochromatosis; hemojuvelin (HJV); hepcidin; hypotransferrinemia; transferrin receptor 2 (TFR2).
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Medical

