A Randomized Trial of Insulin Glargine plus Oral Hypoglycemic Agents versus Continuous Subcutaneous Insulin Infusion to Treat Newly Diagnosed Type 2 Diabetes
- PMID: 30420969
- PMCID: PMC6215559
- DOI: 10.1155/2018/2791584
A Randomized Trial of Insulin Glargine plus Oral Hypoglycemic Agents versus Continuous Subcutaneous Insulin Infusion to Treat Newly Diagnosed Type 2 Diabetes
Abstract
Aims: Basal insulin plus oral hypoglycemic agents (OHAs) has not been investigated for early intensive antihyperglycemic treatment in people with newly diagnosed type 2 diabetes. This study is aimed at comparing the short-term (over a period of 12 days) effects of basal insulin glargine plus OHAs and continuous subcutaneous insulin infusion (CSII) on glycemic control and beta-cell function in this setting.
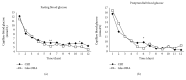
Methods: An open-label parallel-group study. Newly diagnosed hospitalized patients with type 2 diabetes and fasting plasma glucose (FPG) ≥11.1 mmol/L or glycated hemoglobin (HbA1c) ≥9% (75 mmol/mol) were randomized to CSII or insulin glargine in combination with metformin and gliclazide. The primary outcome measure was the mean amplitude of glycemic excursions (MAGE), and secondary endpoints included time to reach glycemic control target (FPG < 7 mmol/L and 2-hour postprandial plasma glucose < 10 mmol/L), markers of β-cell function, and hypoglycemia.
Results: Subjects in the CSII (n = 35) and basal insulin plus OHA (n = 33) groups had a similar significant reduction from baseline to end of treatment in glycated albumin (-6.44 ± 3.23% and- 6.42 ± 3.56%, P = 0.970). Groups A and B have comparable time to glycemic control (3.6 ± 1.2 days and 4.0 ± 1.4 days), MAGE (3.40 ± 1.40 mmol/L vs. 3.16 ± 1.38 mmol/L; p = 0.484), and 24-hour mean blood glucose (7.49 ± 0.96 mmol/L vs. 7.02 ± 1.03 mmol/L). Changes in the C-peptide reactivity index, the secretory unit of islet in transplantation index, and insulin secretion-sensitivity index-2 indicated a greater β-cell function improvement with basal insulin plus OHAs versus CSII.
Conclusions: Short-term insulin glargine plus OHAs may be an alternative to CSII for initial intensive therapy in people with newly diagnosed type 2 diabetes.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical