Non-coordinative metal selectivity bias in human metallothioneins metal-thiolate clusters
- PMID: 30420986
- PMCID: PMC6450656
- DOI: 10.1039/c8mt00264a
Non-coordinative metal selectivity bias in human metallothioneins metal-thiolate clusters
Abstract
Mammalian metallothioneins (MT-1 through MT-4) are a class of metal binding proteins containing two metal-thiolate clusters formed through the preferential coordination of d10 metals, Cu(i) and Zn(ii), by 20 conserved cysteine residues located in two protein domains. MT metalation (homometallic or heterometallic Zn(ii)/Cu(i) species) appears to be isoform specific and controlling zinc and copper concentrations to perform specific and distinct biological functions. Structural and functional relationships, and in vivo metalation studies, identified evolutionary features defining the metal-selectivity nature for MTs. Metallothionein-3 (MT-3) has been shown to possess the most pronounced Cu-thionein character forming Cu(i)-containing species more favorably than metallothionein-2 (MT-2), which possesses the strongest Zn-thionein character. In this work, we identify isoform-specific determinants which control metal binding selectivity bias in different MTs isoforms. By studying the reactivity of Zn7MT-2, Zn7MT-3 and Zn7MT-3 mutants towards Cu(ii) to form Cu(i)4Zn4MTs, we have identified isoform-specific key non-coordinating residues governing folding/outer sphere control of metal selectivity bias in MTs metal clusters. By mutating selected residues and motifs in MT-3 to the corresponding MT-2 amino acids, we dissected key roles in modulating cluster dynamic and metal exchange rates, in increasing the Cu(i)-affinity in MT-3 N-terminal β-domain and/or modulating the higher stability of the Zn(ii)-thiolate cluster in MT-2 β-domain. We thus engineered MT-3 variants in which the copper-thionein character is converted into a zinc-thionein. These results provide new insights into the molecular determinants governing metal selectivity in metal-thiolate clusters.
Conflict of interest statement
CONFLICT OF INTEREST
There are no conflicts of interest to declare.
Figures
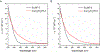
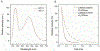

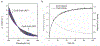



References
-
- Vašák M and Romero-Isart N, in Encyclopedia of Inorganic Chemistry, 2nd edition, ed. K. R. B., J. Wiley & Sons Ltd, New York, 2005, pp. 3208–3221.
-
- Vasak M and Meloni G, Chemistry and biology of mammalian metallothioneins, J. Biol. Inorg. Chem, 2011, 16, 1067–1078. - PubMed
-
- Arseniev A, Schultze P, Wörgötter E, Braun W, Wagner G, Vašák M, Kägi JHR and Wüthrich K, Three-dimensional structure of rabbit liver Cd7metallothionein-2a in aqueous solution determined by nuclear magnetic resonance, J. Mol. Biol, 1988, 201, 637–657. - PubMed
-
- Schultze P, Wörgötter E, Braun W, Wagner G, Vašák M, Kägi JH and Wüthrich K, Conformation of Cd7-metallothionein-2 from rat liver in aqueous solution determined by nuclear magnetic resonance spectroscopy, J. Mol. Biol, 1988, 203, 251–268. - PubMed
-
- Messerle BA, Schäffer A, Vašák M, Kägi JH and Wüthrich K, Three-dimensional structure of human [113Cd7]metallothionein-2 in solution determined by nuclear magnetic resonance spectroscopy, J. Mol. Biol, 1990, 214, 765–779. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

