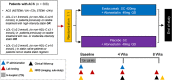
Design of the randomized, placebo-controlled evolocumab for early reduction of LDL-cholesterol levels in patients with acute coronary syndromes (EVOPACS) trial
- PMID: 30421481
- PMCID: PMC6490138
- DOI: 10.1002/clc.23112
Design of the randomized, placebo-controlled evolocumab for early reduction of LDL-cholesterol levels in patients with acute coronary syndromes (EVOPACS) trial
Abstract
Statins lower low-density lipoprotein cholesterol (LDL-C) and improve clinical outcomes in patients with atherosclerotic cardiovascular disease (CVD). Patients with acute coronary syndromes (ACS) often do not achieve LDL-C targets despite potent statin treatment, and have a particularly high risk of early recurrent events. Evolocumab, a proprotein convertase subtilisin/kexin type (PCSK9)-inhibitor resulting in rapid, marked LDL-C reduction, has been studied in hypercholesterolemic subjects without CVD and stabilized patients with CVD; the feasibility, safety, and efficacy of this treatment initiated in the acute phase of ACS remain unknown. We report the design of evolocumab for early reduction of LDL-cholesterol levels in patients with ACS (EVOPACS), a phase-3, multicenter, randomized, double-blind, placebo-controlled trial to assess the feasibility, safety, and LDL-C-lowering efficacy of evolocumab on top of atorvastatin 40 mg in patients with ACS. The primary endpoint is percent change in LDL-C from baseline to 8 weeks. Secondary endpoints are adverse events and serious adverse events. Against a background of beneficial cardiovascular effects of statins beyond LDL-C lowering and in view of preclinical evidence of similar effects of PCSK9 inhibition, the study will also address a variety of exploratory endpoints including the change in C-reactive protein and other inflammatory biomarkers; platelet reactivity; and occurrence of contrast-induced acute kidney injury and myocardial injury in patients undergoing cardiac catheterization. An intracoronary imaging sub-study will investigate the change from baseline in the lipid core burden index in non-culprit lesions, as assessed by serial near-infrared spectroscopy. Recruitment began in January 2018 and enrollment of 308 patients is planned.
Keywords: PCSK9 inhibitor; acute coronary syndrome; lipidology.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
F.M. has received (through the Geneva University Hospital) research grants from Amgen, Sanofi, and MSD for his work in the lipid field. S.W. reports research grants to the institution from Abbott, Amgen, Bayer, Boston Scientific, Biotronik, Medtronic, Edwards Lifesciences, St Jude, Terumo. C.M. has received research grants to institution from Abbott, Beckman Coulter, Biomerieux, Brahms, Roche, Siemens, Singulex, Sphingotec, and speakers/consulting honoraria from Amgen, Astra Zeneca, Boehringer Ingelheim, Duke Research Institute, Roche, Sanofi, Siemens, and Singulex. L.R. received research grants from Sanofi/Regeneron and speaker fees from Sanofi/Regeneron and Amgen. The other authors have no conflict of interest to declare.
Figures
Similar articles
-
Evolocumab for Early Reduction of LDL Cholesterol Levels in Patients With Acute Coronary Syndromes (EVOPACS).J Am Coll Cardiol. 2019 Nov 19;74(20):2452-2462. doi: 10.1016/j.jacc.2019.08.010. Epub 2019 Aug 31. J Am Coll Cardiol. 2019. PMID: 31479722 Clinical Trial.
-
Comparison of PCSK9 Inhibitor Evolocumab vs Ezetimibe in Statin-Intolerant Patients: Design of the Goal Achievement After Utilizing an Anti-PCSK9 Antibody in Statin-Intolerant Subjects 3 (GAUSS-3) Trial.Clin Cardiol. 2016 Mar;39(3):137-44. doi: 10.1002/clc.22518. Epub 2016 Mar 4. Clin Cardiol. 2016. PMID: 26946077 Free PMC article. Clinical Trial.
-
Design and rationale of the GAUSS-2 study trial: a double-blind, ezetimibe-controlled phase 3 study of the efficacy and tolerability of evolocumab (AMG 145) in subjects with hypercholesterolemia who are intolerant of statin therapy.Clin Cardiol. 2014 Mar;37(3):131-9. doi: 10.1002/clc.22248. Epub 2014 Jan 29. Clin Cardiol. 2014. PMID: 24477778 Free PMC article. Clinical Trial.
-
New LDL-cholesterol lowering therapies: pharmacology, clinical trials, and relevance to acute coronary syndromes.Clin Ther. 2013 Aug;35(8):1082-98. doi: 10.1016/j.clinthera.2013.06.019. Epub 2013 Aug 8. Clin Ther. 2013. PMID: 23932550 Review.
-
Management of Hypercholesterolemia, Appropriateness of Therapeutic Approaches and New Drugs in Patients with High Cardiovascular Risk.High Blood Press Cardiovasc Prev. 2016 Sep;23(3):217-30. doi: 10.1007/s40292-016-0155-2. Epub 2016 Aug 27. High Blood Press Cardiovasc Prev. 2016. PMID: 27567901 Free PMC article. Review.
Cited by
-
Insight into the Evolving Role of PCSK9.Metabolites. 2022 Mar 17;12(3):256. doi: 10.3390/metabo12030256. Metabolites. 2022. PMID: 35323699 Free PMC article. Review.
-
Can proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors regress coronary atherosclerotic plaque? A systematic review and meta-analysis.Am J Transl Res. 2023 Jan 15;15(1):452-465. eCollection 2023. Am J Transl Res. 2023. PMID: 36777825 Free PMC article. Review.
-
Effects of Evolocumab Added to Moderate-Intensity Statin Therapy in Chinese Patients With Acute Coronary Syndrome: The EMSIACS Trial Study Protocol.Front Physiol. 2021 Nov 23;12:750872. doi: 10.3389/fphys.2021.750872. eCollection 2021. Front Physiol. 2021. PMID: 34887772 Free PMC article.
-
Impact of PCSK9 Inhibition on Proinflammatory Cytokines and Matrix Metalloproteinases Release in Patients with Mixed Hyperlipidemia and Vulnerable Atherosclerotic Plaque.Pharmaceuticals (Basel). 2022 Jun 27;15(7):802. doi: 10.3390/ph15070802. Pharmaceuticals (Basel). 2022. PMID: 35890100 Free PMC article.
-
Early Management of Blood Lipid Levels with Non-Statin Lipid-Lowering Drugs in Acute Coronary Syndrome: A Mini Review.Cardiovasc Drugs Ther. 2024 Jun 29. doi: 10.1007/s10557-024-07587-9. Online ahead of print. Cardiovasc Drugs Ther. 2024. PMID: 38951453 Review.
References
-
- Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267‐315. - PubMed
-
- Koskinas KC, Siontis GCM, Piccolo R, et al. Effect of statins and non‐statin LDL‐lowering medications on cardiovascular outcomes in secondary prevention: a meta‐analysis of randomized trials. Eur Heart J. 2018;39:1172‐1180. - PubMed
-
- Ray KK, Cannon CP, McCabe CH, et al. Early and late benefits of high‐dose atorvastatin in patients with acute coronary syndromes. Results from the PROVE IT‐TIMI 22 trial. J Am Coll Cardiol. 2005;46:1405‐1410. - PubMed
-
- Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes. JAMA. 2001;285:1711‐1718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous