Increases in controlled-release oxycodone utilisation following the subsidy of oxycodone with naloxone formulations: An Australian population-based study
- PMID: 30421838
- PMCID: PMC6687879
- DOI: 10.1002/pds.4683
Increases in controlled-release oxycodone utilisation following the subsidy of oxycodone with naloxone formulations: An Australian population-based study
Abstract
Purpose: Despite increasing use of oxycodone/naloxone controlled-release (CR) in Australia, little is known about how it has affected the overall oxycodone CR market since its subsidy in 2011.
Methods: We used Pharmaceutical Benefits Scheme dispensing claims (2006-2016) and interrupted time series analysis to examine changes in the quarterly rates of dispensing of oral oxycodone CR formulations (oxycodone/naloxone CR and single-ingredient oxycodone CR) and new oxycodone CR treatment episodes. We also performed a retrospective cohort study in a sample of people initiating a new oxycodone CR treatment episode in 2009, 2012/2013, and 2016 to compare opioid utilisation patterns over time.
Results: The subsidy of oxycodone/naloxone CR was associated with a 1.6-fold increase in the growth rate of oxycodone CR dispensing, resulting from rapid uptake of low strength (≤5 mg) oxycodone/naloxone CR. In our cohort of initiators, the number of new oxycodone CR treatment episodes increased 2.1-fold between 2009 and 2016; in 2016, 91.4% of new treatment episodes involved oxycodone/naloxone CR. Comparing 2016 with 2009, we observed an increase in people initiating with a tablet strength less than or equal to 5-mg (risk difference [RD] = 21.1%, 95% CI, 19.9%-22.4%) in people initiating with no other opioid dispensing 90 days prior to initiation (RD = 5.2%, 3.8%-6.6%) and with no further opioid dispensing 90 days after initiation (RD = 8.8%, 7.4%-10.2%).
Conclusions: After its subsidy, the uptake of low-dose oxycodone/naloxone CR was greater than expected if it were substituting the single-ingredient oxycodone CR, resulting in an expansion of the oxycodone CR market.
Keywords: Australia; drug utilisation; opioids; oxycodone; pharmacoepidemiology.
© 2018 John Wiley & Sons, Ltd.
Conflict of interest statement
Figures
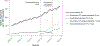
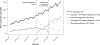
Similar articles
-
Patterns of oxycodone controlled release use in older people with cancer following public subsidy of oxycodone/naloxone formulations: An Australian population-based study.Asia Pac J Clin Oncol. 2021 Feb;17(1):68-78. doi: 10.1111/ajco.13408. Epub 2020 Sep 14. Asia Pac J Clin Oncol. 2021. PMID: 32924282
-
Changes in consumption of opioid analgesics in Israel 2009 to 2016: An update focusing on oxycodone and fentanyl formulations.Pharmacoepidemiol Drug Saf. 2018 May;27(5):535-540. doi: 10.1002/pds.4415. Epub 2018 Feb 28. Pharmacoepidemiol Drug Saf. 2018. PMID: 29488288
-
Person-level changes in oxycodone use after the introduction of a tamper-resistant formulation in Australia.CMAJ. 2018 Mar 26;190(12):E355-E362. doi: 10.1503/cmaj.170666. CMAJ. 2018. PMID: 29581162 Free PMC article.
-
Quality of life and healthcare resource in patients receiving opioids for chronic pain: a review of the place of oxycodone/naloxone.Clin Drug Investig. 2015 Jan;35(1):1-11. doi: 10.1007/s40261-014-0254-6. Clin Drug Investig. 2015. PMID: 25479959 Free PMC article. Review.
-
Oxycodone/Naloxone prolonged-release: a review of its use in the management of chronic pain while counteracting opioid-induced constipation.Drugs. 2014 Mar;74(3):353-75. doi: 10.1007/s40265-014-0177-9. Drugs. 2014. PMID: 24452879 Review.
Cited by
-
National drug utilization trend of analgesics in China: an analysis of procurement data at 793 public hospitals from 2013 to 2018.J Pharm Policy Pract. 2021 May 25;14(1):45. doi: 10.1186/s40545-021-00325-8. J Pharm Policy Pract. 2021. PMID: 34034830 Free PMC article.
-
Duration of opioid use and association with socioeconomic status, daily dose and formulation: a two-decade population study in Queensland, Australia.Int J Clin Pharm. 2021 Apr;43(2):340-350. doi: 10.1007/s11096-020-01079-0. Epub 2020 Jun 16. Int J Clin Pharm. 2021. PMID: 32556897
-
A comparison of educational events for physicians and nurses in Australia sponsored by opioid manufacturers.PLoS One. 2021 Mar 18;16(3):e0248238. doi: 10.1371/journal.pone.0248238. eCollection 2021. PLoS One. 2021. PMID: 33735203 Free PMC article.
References
-
- US Food and Drug Administration. Drug@FDA: FDA Approved Drug Products. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.... 2017. Accessed Oct 10, 2017.
-
- European Medicines Agency. Oxynal-Targin and associated names. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referr.... 2017. Accessed Oct 10, 2017.
-
- Health Canada. Drug Details - Drug and Health Product Register. https://hpr-rps.hres.ca/details.php?drugproductid=2247&query=targin. 2017. Accessed Oct 11, 2017.
-
- Poelaert J, Koopmans-Klein G, Dioh A, et al. Treatment with prolonged-release oxycodone/naloxone improves pain relief and opioid-induced constipation compared with prolonged-release oxycodone in patients with chronic severe pain and laxative-refractory constipation. Clin Ther. 2015;37(4):784–792. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

