A Randomized, Double-Blind, Placebo-Controlled Study of Gelesis100: A Novel Nonsystemic Oral Hydrogel for Weight Loss
- PMID: 30421844
- PMCID: PMC6587502
- DOI: 10.1002/oby.22347
A Randomized, Double-Blind, Placebo-Controlled Study of Gelesis100: A Novel Nonsystemic Oral Hydrogel for Weight Loss
Erratum in
-
Erratum: A Randomized, Double-Blind, Placebo-Controlled Study of Gelesis100: A Novel Nonsystemic Oral Hydrogel for Weight Loss.Obesity (Silver Spring). 2019 Apr;27(4):679. doi: 10.1002/oby.22445. Epub 2019 Feb 12. Obesity (Silver Spring). 2019. PMID: 30900411 Free PMC article. No abstract available.
-
Erratum: A Randomized, Double-Blind, Placebo-Controlled Study of Gelesis100: A Novel Nonsystemic Oral Hydrogel for Weight Loss.Obesity (Silver Spring). 2019 Jul;27(7):1210. doi: 10.1002/oby.22533. Epub 2019 May 13. Obesity (Silver Spring). 2019. PMID: 31231960 Free PMC article. No abstract available.
Abstract
Objective: This study aims to assess the efficacy and safety of Gelesis100, a novel, nonsystemic, superabsorbent hydrogel to treat overweight or obesity.
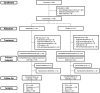
Methods: The Gelesis Loss Of Weight (GLOW) study was a 24-week, multicenter, randomized, double-blind, placebo-controlled study in patients with BMI ≥ 27 and ≤ 40 kg/m2 and fasting plasma glucose ≥ 90 and ≤ 145 mg/dL. The co-primary end points were placebo-adjusted weight loss (superiority and 3% margin super-superiority) and at least 35% of patients in the Gelesis100 group achieving ≥ 5% weight loss.
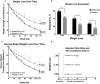
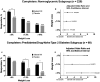
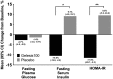
Results: Gelesis100 treatment caused greater weight loss over placebo (6.4% vs. 4.4%, P = 0.0007), achieving 2.1% superiority but not 3% super-superiority. Importantly, 59% of Gelesis100-treated patients achieved weight loss of ≥ 5%, and 27% achieved ≥ 10% versus 42% and 15% in the placebo group, respectively. Gelesis100-treated patients had twice the odds of achieving ≥ 5% and ≥ 10% weight loss versus placebo (adjusted OR: 2.0, P = 0.0008; OR: 2.1, P = 0.0107, respectively), with 5% responders having a mean weight loss of 10.2%. Patients with prediabetes or drug-naive type 2 diabetes had six times the odds of achieving ≥ 10% weight loss. Gelesis100 treatment had no apparent increased safety risks.
Conclusions: Gelesis100 is a promising new nonsystemic therapy for overweight and obesity with a highly desirable safety and tolerability profile.
Trial registration: ClinicalTrials.gov NCT03008954.
© 2018 The Authors. Obesity published by Wiley Periodicals, Inc. on behalf of The Obesity Society (TOS).
Figures
Comment in
-
A Weight Loss Device That Looks Like a Pill.Obesity (Silver Spring). 2019 Feb;27(2):189. doi: 10.1002/oby.22399. Obesity (Silver Spring). 2019. PMID: 30677264 No abstract available.
-
Unclear Benefit of Gelesis100 for Body Weight Loss.Obesity (Silver Spring). 2019 Sep;27(9):1383. doi: 10.1002/oby.22532. Epub 2019 Jul 2. Obesity (Silver Spring). 2019. PMID: 31264776 No abstract available.
-
Response to "Unclear Benefit of Gelesis100 for Body Weight Loss".Obesity (Silver Spring). 2019 Sep;27(9):1384. doi: 10.1002/oby.22530. Epub 2019 Jul 2. Obesity (Silver Spring). 2019. PMID: 31264796 No abstract available.

