Survival Outcomes in Patients With Previously Untreated BRAF Wild-Type Advanced Melanoma Treated With Nivolumab Therapy: Three-Year Follow-up of a Randomized Phase 3 Trial
- PMID: 30422243
- PMCID: PMC6439558
- DOI: 10.1001/jamaoncol.2018.4514
Survival Outcomes in Patients With Previously Untreated BRAF Wild-Type Advanced Melanoma Treated With Nivolumab Therapy: Three-Year Follow-up of a Randomized Phase 3 Trial
Erratum in
-
Incorrect Treatment Group in Results and Error in Legend of Figure 2.JAMA Oncol. 2019 Feb 1;5(2):271. doi: 10.1001/jamaoncol.2018.6113. JAMA Oncol. 2019. PMID: 30520938 Free PMC article. No abstract available.
Abstract
Importance: This analysis provides long-term follow-up in patients with BRAF wild-type advanced melanoma receiving first-line therapy based on anti-programmed cell death 1 receptor inhibitors.
Objective: To compare the 3-year survival with nivolumab vs that with dacarbazine in patients with previously untreated BRAF wild-type advanced melanoma.
Design, setting, and participants: This follow-up of a randomized phase 3 trial analyzed 3-year overall survival data from the randomized, controlled, double-blind CheckMate 066 phase 3 clinical trial. For this ongoing, multicenter academic institution trial, patients were enrolled from January 2013 through February 2014. Eligible patients were 18 years or older with confirmed unresectable previously untreated stage III or IV melanoma and an Eastern Cooperative Oncology Group performance status of 0 or 1 but without a BRAF mutation.
Interventions: Patients were treated until progression or unacceptable toxic events with nivolumab (3 mg/kg every 2 weeks plus dacarbazine-matched placebo every 3 weeks) or dacarbazine (1000 mg/m2 every 3 weeks plus nivolumab-matched placebo every 2 weeks).
Main outcome and measure: Overall survival.
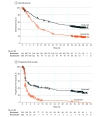
Results: At minimum follow-ups of 38.4 months among 210 participants in the nivolumab group (median age, 64 years [range, 18-86 years]; 57.6% male) and 38.5 months among 208 participants in the dacarbazine group (median age, 66 years [range, 25-87 years]; 60.1% male), 3-year overall survival rates were 51.2% (95% CI, 44.1%-57.9%) and 21.6% (95% CI, 16.1%-27.6%), respectively. The median overall survival was 37.5 months (95% CI, 25.5 months-not reached) in the nivolumab group and 11.2 months (95% CI, 9.6-13.0 months) in the dacarbazine group (hazard ratio, 0.46; 95% CI, 0.36-0.59; P < .001). Complete and partial responses, respectively, were reported for 19.0% (40 of 210) and 23.8% (50 of 210) of patients in the nivolumab group compared with 1.4% (3 of 208) and 13.0% (27 of 208) of patients in the dacarbazine group. Additional analyses were performed on outcomes with subsequent therapies. Treatment-related grade 3/4 adverse events occurred in 15.0% (31 of 206) of nivolumab-treated patients and in 17.6% (36 of 205) of dacarbazine-treated patients. There were no deaths due to study drug toxic effects.
Conclusions and relevance: Nivolumab led to improved 3-year overall survival vs dacarbazine in patients with previously untreated BRAF wild-type advanced melanoma.
Trial registration: ClinicalTrials.gov identifier: NCT01721772.
Conflict of interest statement
Figures
References
-
- Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti–CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375-384. doi: 10.1016/S1470-2045(15)70076-8 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

