Machine learning assessment of myocardial ischemia using angiography: Development and retrospective validation
- PMID: 30422987
- PMCID: PMC6233920
- DOI: 10.1371/journal.pmed.1002693
Machine learning assessment of myocardial ischemia using angiography: Development and retrospective validation
Abstract
Background: Invasive fractional flow reserve (FFR) is a standard tool for identifying ischemia-producing coronary stenosis. However, in clinical practice, over 70% of treatment decisions still rely on visual estimation of angiographic stenosis, which has limited accuracy (about 60%-65%) for the prediction of FFR < 0.80. One of the reasons for the visual-functional mismatch is that myocardial ischemia can be affected by the supplied myocardial size, which is not always evident by coronary angiography. The aims of this study were to develop an angiography-based machine learning (ML) algorithm for predicting the supplied myocardial volume for a stenosis, as measured using coronary computed tomography angiography (CCTA), and then to build an angiography-based classifier for the lesions with an FFR < 0.80 versus ≥ 0.80.
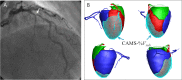
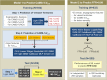
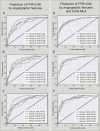
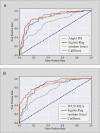
Methods and findings: A retrospective study was conducted using data from 1,132 stable and unstable angina patients with 1,132 intermediate lesions who underwent invasive coronary angiography, FFR, and CCTA at the Asan Medical Center, Seoul, Korea, between 1 May 2012 and 30 November 2015. The mean age was 63 ± 10 years, 76% were men, and 72% of the patients presented with stable angina. Of these, 932 patients (assessed before 31 January 2015) constituted the training set for the algorithm, and 200 patients (assessed after 1 February 2015) served as a test cohort to validate its diagnostic performance. Additionally, external validation with 79 patients from two centers (CHA University, Seongnam, Korea, and Ajou University, Suwon, Korea) was conducted. After automatic contour calibration using the caliber of guiding catheter, quantitative coronary angiography was performed using the edge-detection algorithms (CAAS-5, Pie-Medical). Clinical information was provided by the Asan BiomedicaL Research Environment (ABLE) system. The CCTA-based myocardial segmentation (CAMS)-derived myocardial volume supplied by each vessel (right coronary artery [RCA], left anterior descending [LAD], left circumflex [LCX]) and the myocardial volume subtended to a stenotic segment (CAMS-%Vsub) were measured for labeling. The ML for (1) predicting vessel territories (CAMS-%LAD, CAMS-%LCX, and CAMS-%RCA) and CAMS-%Vsub and (2) identifying the lesions with an FFR < 0.80 was constructed. Angiography-based ML, employing a light gradient boosting machine (GBM), showed mean absolute errors (MAEs) of 5.42%, 8.57%, and 4.54% for predicting CAMS-%LAD, CAMS-%LCX, and CAMS-%RCA, respectively. The percent myocardial volumes predicted by ML were used to predict the CAMS-%Vsub. With 5-fold cross validation, the MAEs between ML-predicted percent myocardial volume subtended to a stenotic segment (ML-%Vsub) and CAMS-%Vsub were minimized by the elastic net (6.26% ± 0.55% for LAD, 5.79% ± 0.68% for LCX, and 2.95% ± 0.14% for RCA lesions). Using all attributes (age, sex, involved vessel segment, and angiographic features affecting the myocardial territory and stenosis degree), the ML classifiers (L2 penalized logistic regression, support vector machine, and random forest) predicted an FFR < 0.80 with an accuracy of approximately 80% (area under the curve [AUC] = 0.84-0.87, 95% confidence intervals 0.71-0.94) in the test set, which was greater than that of diameter stenosis (DS) > 53% (66%, AUC = 0.71, 95% confidence intervals 0.65-0.78). The external validation showed 84% accuracy (AUC = 0.89, 95% confidence intervals 0.83-0.95). The retrospective design, single ethnicity, and the lack of clinical outcomes may limit this prediction model's generalized application.
Conclusion: We found that angiography-based ML is useful to predict subtended myocardial territories and ischemia-producing lesions by mitigating the visual-functional mismatch between angiographic and FFR. Assessment of clinical utility requires further validation in a large, prospective cohort study.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107:2900–2907. 10.1161/01.CIR.0000072790.23090.41 - DOI - PubMed
-
- Tonino PA Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, Ver Lee PN, et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. 2010;55:2816–2821. 10.1016/j.jacc.2009.11.096 - DOI - PubMed
-
- Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Maron DJ, et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2017;69:2212–2241. 10.1016/j.jacc.2017.02.001 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

