Loss of function of NF1 is a mechanism of acquired resistance to endocrine therapy in lobular breast cancer
- PMID: 30423024
- PMCID: PMC6336006
- DOI: 10.1093/annonc/mdy497
Loss of function of NF1 is a mechanism of acquired resistance to endocrine therapy in lobular breast cancer
Abstract
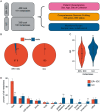
Background: Invasive lobular carcinoma (ILC) as a disease entity distinct from invasive ductal carcinoma (IDC) has merited focused studies of the genomic landscape, but those to date are largely limited to the assessment of early-stage cancers. Given that genomic alterations develop as acquired resistance to endocrine therapy, studies on refractory ILC are needed.
Patients and methods: Tissue from 336 primary-enriched, breast-biopsied ILC and 485 estrogen receptor (ER)-positive IDC and metastatic biopsy specimens from 180 ILC and 191 ER-positive IDC patients was assayed with hybrid-capture-based comprehensive genomic profiling for short variant, indel, copy number variants, and rearrangements in up to 395 cancer-related genes.
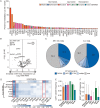
Results: Whereas ESR1 alterations are enriched in the metastases of both ILC and IDC compared with breast specimens, NF1 alterations are enriched only in ILC metastases (mILC). NF1 alterations are predominantly under loss of heterozygosity (11/14, 79%), are mutually exclusive with ESR1 mutations [odds ratio = 0.24, P < 0.027] and are frequently polyclonal in ctDNA assays. Assessment of paired specimens shows that NF1 alterations arise in the setting of acquired resistance. An in vitro model of CDH1 mutated ER-positive breast cancer demonstrates that NF1 knockdown confers a growth advantage in the presence of 4-hydroxy tamoxifen. Our study further identified a significant increase in tumor mutational burden (TMB) in mILCs relative to breast ILCs or metastatic IDCs (8.9% >20 mutations/mb; P < 0.001). Most TMB-high mILCs harbor an APOBEC trinucleotide signature (14/16; 88%).
Conclusions: This study identifies alteration of NF1 as enriched specifically in mILC. Mutual exclusivity with ESR1 alterations, polyclonality in relapsed ctDNA, and de novo acquisition suggest a role for NF1 loss in endocrine therapy resistance. Since NF1 loss leads to RAS/RAF kinase activation, patients may benefit from a matched inhibitor. Moreover, for an independent subset of mILC, TMB was elevated relative to breast ILC, suggesting possible benefit from immune checkpoint inhibitors.
Figures


References
-
- Rakha EA, El-Sayed ME, Powe DG. et al. Invasive lobular carcinoma of the breast: response to hormonal therapy and outcomes. Eur J Cancer 2008; 44(1): 73–83. - PubMed
-
- Sikora MJ, Jankowitz RC, Dabbs DJ, Oesterreich S.. Invasive lobular carcinoma of the breast: patient response to systemic endocrine therapy and hormone response in model systems. Steroids 2013; 78(6): 568–575. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

