Semantic Disease Gene Embeddings (SmuDGE): phenotype-based disease gene prioritization without phenotypes
- PMID: 30423077
- PMCID: PMC6129260
- DOI: 10.1093/bioinformatics/bty559
Semantic Disease Gene Embeddings (SmuDGE): phenotype-based disease gene prioritization without phenotypes
Abstract
Motivation: In the past years, several methods have been developed to incorporate information about phenotypes into computational disease gene prioritization methods. These methods commonly compute the similarity between a disease's (or patient's) phenotypes and a database of gene-to-phenotype associations to find the phenotypically most similar match. A key limitation of these methods is their reliance on knowledge about phenotypes associated with particular genes which is highly incomplete in humans as well as in many model organisms such as the mouse.
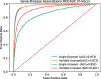
Results: We developed SmuDGE, a method that uses feature learning to generate vector-based representations of phenotypes associated with an entity. SmuDGE can be used as a trainable semantic similarity measure to compare two sets of phenotypes (such as between a disease and gene, or a disease and patient). More importantly, SmuDGE can generate phenotype representations for entities that are only indirectly associated with phenotypes through an interaction network; for this purpose, SmuDGE exploits background knowledge in interaction networks comprised of multiple types of interactions. We demonstrate that SmuDGE can match or outperform semantic similarity in phenotype-based disease gene prioritization, and furthermore significantly extends the coverage of phenotype-based methods to all genes in a connected interaction network.
Availability and implementation: https://github.com/bio-ontology-research-group/SmuDGE.
Figures





References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

