Inhibition of Amino Acid Metabolism Selectively Targets Human Leukemia Stem Cells
- PMID: 30423294
- PMCID: PMC6280965
- DOI: 10.1016/j.ccell.2018.10.005
Inhibition of Amino Acid Metabolism Selectively Targets Human Leukemia Stem Cells
Erratum in
-
Inhibition of Amino Acid Metabolism Selectively Targets Human Leukemia Stem Cells.Cancer Cell. 2019 Feb 11;35(2):333-335. doi: 10.1016/j.ccell.2019.01.013. Cancer Cell. 2019. PMID: 30753831 Free PMC article. No abstract available.
Abstract
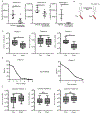
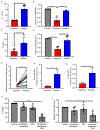
In this study we interrogated the metabolome of human acute myeloid leukemia (AML) stem cells to elucidate properties relevant to therapeutic intervention. We demonstrate that amino acid uptake, steady-state levels, and catabolism are all elevated in the leukemia stem cell (LSC) population. Furthermore, LSCs isolated from de novo AML patients are uniquely reliant on amino acid metabolism for oxidative phosphorylation and survival. Pharmacological inhibition of amino acid metabolism reduces oxidative phosphorylation and induces cell death. In contrast, LSCs obtained from relapsed AML patients are not reliant on amino acid metabolism due to their ability to compensate through increased fatty acid metabolism. These findings indicate that clinically relevant eradication of LSCs can be achieved with drugs that target LSC metabolic vulnerabilities.
Keywords: acute myeloid leukemia; amino acids; cancer cell metabolism; leukemia stem cells; metabolomics; venetoclax.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures






Comment in
-
Metabolic Flexibility in Leukemia-Adapt or Die.Cancer Cell. 2018 Nov 12;34(5):695-696. doi: 10.1016/j.ccell.2018.10.012. Cancer Cell. 2018. PMID: 30423291
References
-
- Dick JE (2005). Acute myeloid leukemia stem cells. Ann N Y Acad Sci 1044, 1–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

