Erythrocytic bioactivation of nitrite and its potentiation by far-red light
- PMID: 30423533
- PMCID: PMC6230921
- DOI: 10.1016/j.redox.2018.11.001
Erythrocytic bioactivation of nitrite and its potentiation by far-red light
Abstract
Background: Nitrite is reduced by heme-proteins and molybdenum-containing enzymes to form the important signaling molecule nitric oxide (NO), mediating NO signaling. Substantial evidence suggests that deoxygenated hemoglobin within red blood cells (RBCs) is the main erythrocytic protein responsible for mediating nitrite-dependent NO signaling. In other work, infrared and far red light have been shown to have therapeutic potential that some attribute to production of NO. Here we explore whether a combination of nitrite and far red light treatment has an additive effect in NO-dependent processes, and whether this effect is mediated by RBCs.
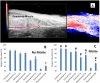
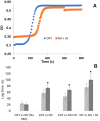
Methods and results: Using photoacoustic imaging in a rat model as a function of varying inspired oxygen, we found that far red light (660 nm, five min. exposure) and nitrite feeding (three weeks in drinking water at 100 mg/L) each separately increased tissue oxygenation and vessel diameter, and the combined treatment was additive. We also employed inhibition of human platelet activation measured by flow cytometry to assess RBC-dependent nitrite bioactivation and found that far red light dramatically potentiates platelet inhibition by nitrite. Blocking RBC-surface thiols abrogated these effects of nitrite and far-red light. RBC-dependent production of NO was also shown to be enhanced by far red light using a chemiluminescence-based nitric oxide analyzer. In addition, RBC-dependent bioactivation of nitrite led to prolonged lag times for clotting in platelet poor plasma that was enhanced by exposure to far red light.
Conclusions: Our results suggest that nitrite leads to the formation of a photolabile RBC surface thiol-bound species such as an S-nitrosothiol or heme-nitrosyl (NO-bound heme) for which far red light enhances NO signaling. These findings expand our understanding of RBC-mediated NO production from nitrite. This pathway of NO production may have therapeutic potential in several applications including thrombosis, and, thus, warrants further study.
Keywords: Hemoglobin; Light therapy; Nitric oxide; Nitrite; Photobiomodulation; Red blood cells.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures





References
-
- Ignarro L.J., Byrns R.E., Buga G.M., Wood K.S. Endothelium-derived relaxing factor from pulmonary-artery and vein possesses pharmacological and chemical-properties identical to those of nitric-oxide radical. Circ. Res. 1987;61(6):866–879. - PubMed
-
- Palmer R.M.J., Ferrige A.G., Moncada S. Nitric-oxide release accounts for the biological-activity of endothelium-derived relaxing factor. Nature. 1987;327(6122):524–526. - PubMed
-
- Furchgott R.F. Studies on relaxation of rabbit aorta by sodium nitrite: the basis for the proposal that the acid- activatable factor from bovine retractor penis is inorganic nitrite and the endothelium-derived relaxing factor is nitric oxide. In: Vanhoutte P.M., editor. Vasodilatation: Vascular Smooth Muscle, Peptides, Autonomic Nerves, and Endothelium. Raven Press; New York: 1988. pp. 401–414.
-
- Ignarro L.J., Byrns R.E., Wood K.S. Biochemical and pharmacological properties of endothelium-derived relaxing factor and its similarity to nitric oxide radical. In: Vanhoutte P.M., editor. Vasodilatation: Vascular Smooth Muscle, Peptides, Autonomic Nerves, and Endothelium. Raven Press; New York: 1988. pp. 427–435.
-
- Loscalzo J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ. Res. 2001;88(8):756–762. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

