Interferons and Dry Eye in Sjögren's Syndrome
- PMID: 30423813
- PMCID: PMC6274689
- DOI: 10.3390/ijms19113548
Interferons and Dry Eye in Sjögren's Syndrome
Abstract
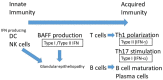
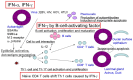
Various cytokines, including interferon (IFN)-γ and IL-17, are augmented, and autoreactive T cells and B cells are activated in the immune pathogenesis of Sjögren's syndrome (SS). In particular, IFNs are involved in both the early stages of innate immunity by high level of type I IFN in glandular tissue and sera and the later stages of disease progression by type I and type II IFN producing T cells and B cells through B cell activating factor in SS. Genetically modified mouse models for some of these molecules have been reported and will be discussed in this review. New findings from human SS and animal models of SS have elucidated some of the mechanisms underlying SS-related dry eye. We will discuss IFN-γ and several other molecules that represent candidate targets for treating inflammation in SS-related dry eye.
Keywords: Interferon-γ; Sjögren’s syndrome; animal model; dry eye.
Conflict of interest statement
The founding sponsors had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Tsuboi H., Hagiwara S., Asashima H., Takahashi H., Hirota T., Noma H., Umehara H., Kawakami A., Nakamura H., Sano H., et al. Comparison of performance of the 2016 ACR-EULAR classification criteria for primary Sjögren’s syndrome with other sets of criteria in Japanese patients. Ann. Rheum. Dis. :2017. doi: 10.1136/annrheumdis-2016-210758. - DOI - PMC - PubMed
-
- Shiboski C.H., Shiboski S.C., Seror R., Criswell L.A., Labetoulle M., Lietman T.M., Rasmussen A., Scofield H., Vitali C., Bowman S.J., et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts. Ann. Rheum. Dis. 2017;76:9–16. doi: 10.1136/annrheumdis-2016-210571. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

