Anticancer Activity of Natural Compounds from Plant and Marine Environment
- PMID: 30423952
- PMCID: PMC6275022
- DOI: 10.3390/ijms19113533
Anticancer Activity of Natural Compounds from Plant and Marine Environment
Abstract
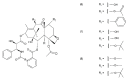
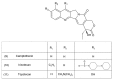
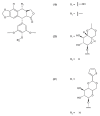
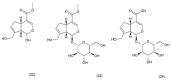
This paper describes the substances of plant and marine origin that have anticancer properties. The chemical structure of the molecules of these substances, their properties, mechanisms of action, their structure⁻activity relationships, along with their anticancer properties and their potential as chemotherapeutic drugs are discussed in this paper. This paper presents natural substances from plants, animals, and their aquatic environments. These substances include the vinca alkaloids, mistletoe plant extracts, podophyllotoxin derivatives, taxanes, camptothecin, combretastatin, and others including geniposide, colchicine, artesunate, homoharringtonine, salvicine, ellipticine, roscovitine, maytanasin, tapsigargin, and bruceantin. Compounds (psammaplin, didemnin, dolastin, ecteinascidin, and halichondrin) isolated from the marine plants and animals such as microalgae, cyanobacteria, heterotrophic bacteria, invertebrates (e.g., sponges, tunicates, and soft corals) as well as certain other substances that have been tested on cells and experimental animals and used in human chemotherapy.
Keywords: anticancer properties; natural compounds; substances from marin.
Conflict of interest statement
The authors declare no conflict of interest. We confirm all figures and tables in your manuscript are non-published and original.
Figures








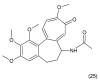
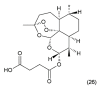
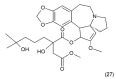

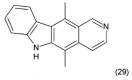


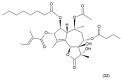
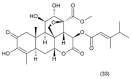

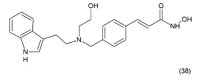
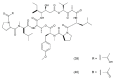
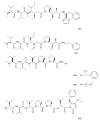

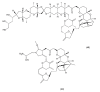
References
-
- Chekem L., Wierucki S. Extraction of artemisinin and synthesis of its derivates artesunate and artemether. Med. Trop. 2006;66:602–605. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

